27 Feb 2017
Helminth control programmes for equine yearlings at pasture
Jacqueline Matthews explores measures to prevent or manage parasitic worms in young horses, with possible treatment options.

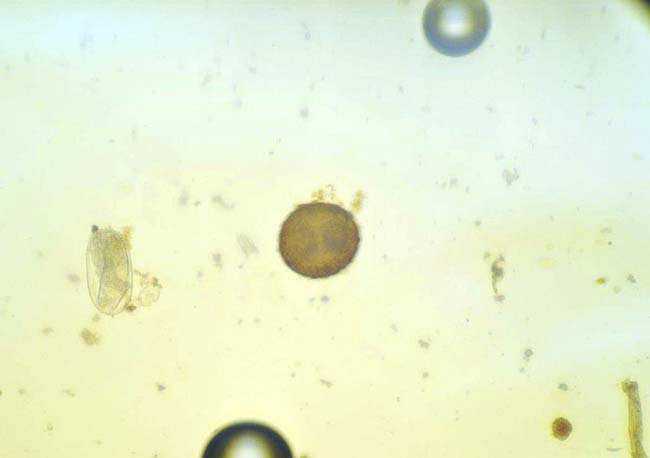
Strongyloides westeri.
Effective parasite control is one of the most important aspects of managing young horses. Grazing horses are exposed to helminths throughout their lives, which can be a particular issue in foals and yearlings due to their higher susceptibility.
Most adult horses control their worm burden well and are at lower risk of developing disease. Higher burdens and clinical symptoms are more likely to occur in foals and adolescent horses. The main aim of control is to limit infection levels in the environment by applying effective anthelmintic treatments in combination with management practices that reduce contamination. It is impossible to eradicate all worms from all horses and attempting to do so will only select for anthelmintic resistance.
This article provides an overview of worms that may be encountered in yearlings and how sustainable control can be approached in this class of stock.
Worms encountered in yearlings at pasture
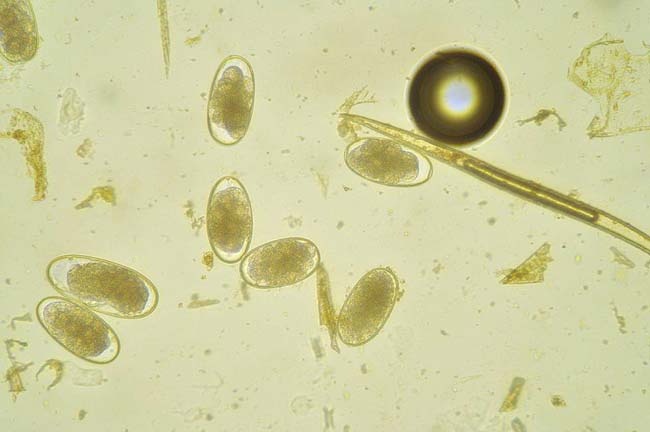
Parascaris equorum is an important roundworm in older foals and yearlings. Infective larvae within resistant, thick-shelled eggs persist for relatively long periods on pasture. After ingestion, larvae hatch and migrate through the liver and lungs to the small intestine. Here, they grow to adults about 10 weeks after infection, with females producing high numbers of eggs.
Immunity is presumed to be age-associated and, by 18 to 24 months, most horses have resistance to infection. Infection can lead to respiratory or intestinal signs, which can occur in the absence of eggs in faeces due to the relatively long prepatent period. Prognosis is good with early diagnosis and effective anthelmintics given; however, prognosis is poor if animals present with acute colic.
All three broad-spectrum anthelmintic classes – benzimidazoles, pyrantel salts and macrocyclic lactones – are licensed against P equorum. These have varying efficacy against larval stages, with macrocyclic lactones exhibiting the highest potency. Of concern, resistance has been reported against macrocyclic lactones; this is at such levels efficacy monitoring should be undertaken annually.
Large and small strongyles can pose problems in adolescent horses. The most important large strongyle species is Strongylus vulgaris; this generally has low prevalence because of high usage of macrocyclic lactones since the 1980s. Anthelmintic resistance has not yet been reported in S vulgaris. Because of its potential pathogenicity (the larvae that live in blood vessels can cause severe colic), it is important S vulgaris is taken into account in control programmes. Studies in Denmark, where anthelmintic usage has reduced considerably due to prescribing regulations, indicate the local prevalence of this worm has increased.
Small strongyles (cyathostomins) are the most prevalent worms. The life cycle involves encystment of larvae in the gut wall; these can persist for months and accumulate in substantial numbers. In the UK, encysted larvae are most usually found in autumn/winter. They can emerge en masse to cause a potentially fatal colitis, most commonly observed in one to three-year-olds.
Anthelmintic resistance is a major issue, particularly against benzimidazoles; in some regions (such as the UK) resistance to this class is ubiquitous. Cyathostomin resistance has also been recorded against pyrantel compounds and an early indicator of resistance (reduced strongyle egg reappearance period after treatment) has been reported for ivermectin and moxidectin. All three broad-spectrum classes are licensed for use against strongyles – these have varying levels of efficacy against larvae, with higher potency observed with macrocyclic lactones, particularly moxidectin.
For the aforementioned species, faecal egg count (FEC) analysis can be used to detect patent infection. This is a valuable tool for monitoring egg shedding and anthelmintic efficacy. FEC tests do not provide information on the presence of larvae. A blood test designed to detect encysted cyathostomins is being developed and will become available in the next two years.
Horses of all ages are susceptible to Oxyuris equi (pinworm), which lives in the rectum. Eggs deposited by females around the anus and tail head cause irritation. In some horses, clinical signs occur; from mild irritation to intense tail rubbing/skin damage.
When O equi is detected, anthelmintic should be administered and individuals washed daily around the perineum/perianal region with an approved detergent. Washing materials must be discarded or cleaned in hot soapy water. Stable walls, gates and fence posts must be cleaned with an equine-safe disinfectant then rinsed.
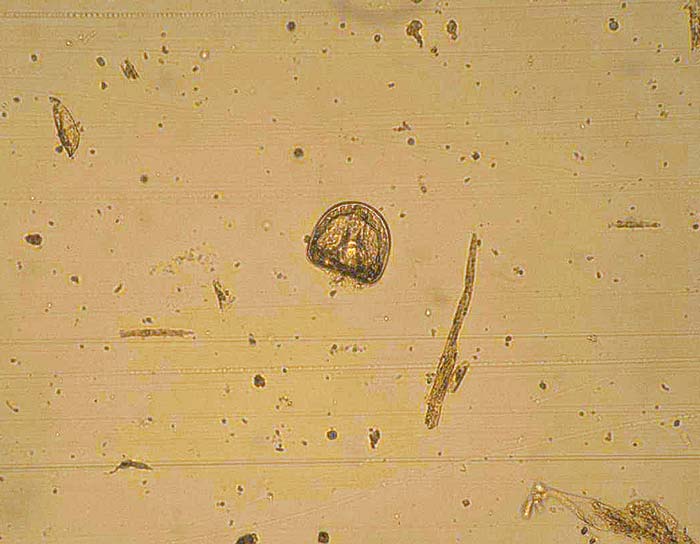
Anoplocephala perfoliata is a common tapeworm with an indirect life cycle involving a mite intermediate host. Prevalence rates vary across regions – the limited epidemiological data available suggests a higher density of mites in moist environments. A perfoliata has been associated with colic and this has been linked to level of burden. Horses of all ages are susceptible, but most have relatively few worms and are unlikely to develop clinical signs.
Diagnosis is complicated as egg shedding is intermittent and standard FEC tests are insensitive. Blood and saliva tests – based on worm-specific antibody – are available in the UK. These can be used to inform treatment decisions, but it must be kept in mind they provide information on exposure to infection and horses can remain test-positive for some time after treatment.
Where these tests are not available, because of A perfoliata’s seasonality and potential to cause disease, a single annual treatment (late autumn/winter) with praziquantel or a pyrantel salt (at twice the dose recommended for nematodes) is advised to mitigate clinical consequences of infection and reduce transmission. No evidence exists that frequent treatments provide a health benefit and no published reports exist of anthelmintic resistance.
Control strategies for yearlings at pasture
Because of the risk of anthelmintic resistance, “non-essential” treatments should be avoided. Younger stock have higher susceptibility to worms and will be more predisposed to disease, as well as have higher egg shedding. Thus, the advantage of reducing selection pressure for resistance by using FEC-directed treatments needs to be balanced against this when managing worms in yearlings.
Programme design must take into account:
- grazing and stock management
- clinical history
- previous anthelmintic usage and efficacy
- diagnostic test results
The programme must be based on knowledge of individual farm practices to allow assessment of transmission and should aim to combine judicious use of anthelmintics with:
- Regular – ideally, twice-weekly – dung removal. Dung must not be disposed on to grazing. Harrowing is not recommended where conditions are wet (most of the UK), as eggs/larvae are unlikely to desiccate after spreading. Pastures used for foals and yearlings must be prioritised for clean management.
- Avoid using the same pastures year on year for foals and yearlings. Mixed or rotational grazing with ruminants can be done. Note liver fluke can be transmitted from ruminants. This worm can cause disease in horses and is difficult to detect. Therefore, if using ruminants, their liver fluke status must be established – especially if grazing in wet, boggy pastures, which support snail intermediate hosts. If ruminants are infected with fluke, ensure treatments are effective.
- Resting pastures to reduce worm contamination. Paddocks need to be rested for at least four to five months (in the UK, ideally January to June) for this to be effective.
- Avoid high stocking density and over-grazing so horses are not forced to ingest pasture close to where dung has been deposited.
These measures can be combined with the following types of treatments:
- all-group therapy to target pathogenic larvae not detectable by diagnostics
- therapy to reduce contamination using FEC testing to identify horses shedding strongyle eggs above a threshold
Recommended treatment thresholds lie in the range of 100 to 500 eggs per gram (EPG). This should be set on a case-by-case basis depending on likely risk of contamination (such as stocking density, frequency/effectiveness of dung removal, shedding patterns and clinical history). No guidelines are available for an EPG threshold for P equorum; if eggs are detected, treatment is recommended. In the UK, treatments can be based on FEC test results from spring till autumn and the testing interval depends on the last anthelmintic used.
The frequency of FEC monitoring should be higher in yearlings than for adults and testing should be done near the expected egg reappearance period for the last anthelmintic used (12 weeks for moxidectin, 8 weeks for ivermectin, and 6 weeks for pyrantel and fenbendazole, if used) as opposed to waiting a few weeks for egg counts to rise. This is because strongyle FECs are likely to rise faster in yearlings after treatment.
High shedding in many individuals at the expected egg reappearance period is suggestive of reduced effectiveness. A FEC reduction test should be performed with the same anthelmintic to confirm sensitivity status.
It is important efficacy testing is performed for strongyle and P equorum infections; there is no point in using anthelmintics that are not effective. Ideally, an annual FEC reduction test should be undertaken. This involves an extra FEC test two weeks after treatment (best done in mid-summer when FECs are likely to be higher on the day of treatment). The percentage reduction in egg count is calculated by comparing the mean FEC of the group before and after treatment.
On farms where ivermectin and fenbendazole efficacy against P equorum has not been examined, checks should be considered. Should resistance to these anthelmintics be identified, pyrantel is the remaining option; however, anecdotal reports exist of resistance to this anthelmintic and multi-class resistance could become a major issue.
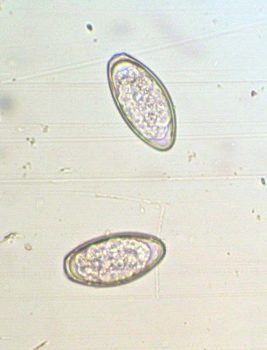
Because strongyle larvae are not detectable by FEC analysis, all-group “larvicidal” treatments should be administered in late autumn/early winter. Most anthelmintics are not effective against inhibited cyathostomin larvae. A five-day course of fenbendazole has been shown to be effective against benzimidazole-sensitive worms, but resistance is high in many regions. This regime is no longer recommended unless the benzimidazole sensitivity of the population has been demonstrated. Moxidectin has licensed effectiveness against all cyathostomin stages as well as large strongyles.
Resistance is not yet commonplace. For this reason, moxidectin is the treatment of choice. In yearlings, a late winter FEC test is recommended to monitor egg shedding, especially if horses have been grazing outside and the weather has been mild. A second moxidectin treatment may be warranted at this time if egg shedding is identified and transmission likely.
New arrivals that will graze with permanent residents should be treated with moxidectin (with or without praziquantel) on admission and kept off pasture, ideally for three days. A FEC test can be performed 14 days later to check effectiveness of the treatment.
Most farms will have horses of different ages, so an integrated control programme that covers all stock types should be drawn up and reviewed at the start of each season. This will ensure risks have been assessed and suitable mitigating measures incorporated into the control programme.
Acknowledgement
Thanks to Dave Bartley, from Moredun Research Institute, for the egg images.
Latest news

Small animal
Expert Insights: The role of monoclonal antibodies (mAbs) in osteoarthritis (OA) management for pets
Sponsored
10 Mar 2025