23 Feb 2015
Managing postoperative pain in companion animals

The major underpinning principles of effective postoperative pain management are attention to pre and intraoperative pain management and robust pain assessment in the postoperative period. Thus postoperative analgesia should be a continuation of intraoperative analgesia strategies adjusted on the basis of pain assessment.
It is important to embrace the concept of “preventive analgesia” throughout the entire perioperative period.
Preventive analgesia, the principle of preventing upregulation of pain pathways through the development of central and peripheral sensitisation (Katz et al, 2011), relies on administering analgesics early and continuing them for an adequate period of time while tissue healing occurs.
Unfortunately, there are very few data on the optimal length of time for analgesic administration after surgery in companion animals. Duration of analgesia administration after surgery is a balance between adequate pain relief and the risk of unnecessary side effects due to too prolonged administration of drugs.
Most studies suggest pain is greatest in the first 24 hours after surgery (Brondani et al, 2009; Linton et al, 2012). However, studies in dogs and cats undergoing ovariohysterectomy suggest overt behavioural changes are present for at least three days after surgery (Fox et al, 2000; Brondani et al, 2009), indicating a minimum of three days’ pain relief is required after this procedure.
Data for other types of procedure are lacking, and this is an area where further research is required.
Multimodal analgesia is also a prerequisite for adequate intra and postoperative pain relief (Slingsby et al, 2001; Slingsby et al, 2010; Brondani et al, 2009), although data in dogs and cats suggests multimodal analgesia strategies (such as administration of an opioid combined with an NSAID) are widely adopted for routine surgeries in the UK (Hunt et al, 2014a).
The focus of this article is pharmacological management of postoperative pain in cats and dogs.
Non-pharmacological therapies – for example, good nursing care or application of heat or cold – can also play a useful adjunctive role in pain management, but are beyond the scope of this article.
Pain assessment
Regular pain assessment (Figure 1) in the postoperative period is pivotal to effective pain management, as without assessment it is impossible to predict whether pain management is adequate and therefore avoid either over or under administration of analgesic drugs. Inadequate analgesia leads to suffering, but over analgesia is also disadvantageous.
For example, excessive opioid administration can lead to prolonged sedation and recumbency that can predispose to respiratory obstruction (for example, in brachycephalic breeds) and reduce appetite and water intake, which is detrimental to recovery from surgery.
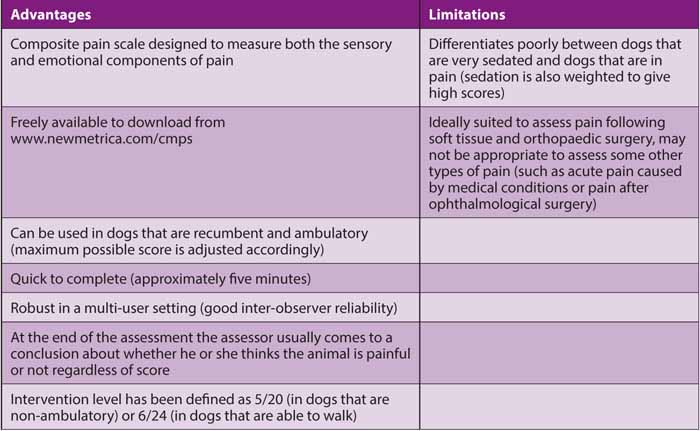
Use of a tool to quantify or score pain can be very helpful postoperatively to track changes in pain level over time and in response to analgesic drug administration. Pain scoring tools can also be very helpful to guide when additional “rescue” analgesia is needed, and validated pain scoring tools in dogs, notably the Glasgow Composite Pain Scale (Holton et al, 2001; Morton et al, 2005; Murrell et al, 2008), and for cats the Botucatu Multidimensional Composite Pain Scale (Brondani et al, 2011 and 2013) have set intervention levels, scores above which administration of further analgesia is recommended.
The Botucatu scale has only recently been validated and has many advantages compared to the Colorado Acute Pain Scale, which is also widely used in cats. For example, the Botucatu scale is validated, more sensitive than the Colorado Scale (maximum possible score is 30 compared with four) and an intervention level has been defined for the Botucatu scale, whereas no intervention level has been validated for the Colorado Scale.
It is difficult to be prescriptive about how often pain should be scored after surgery; the frequency needs to be tailored to meet the demands of the individual patient. However, it is good practice to routinely score pain before the administration of analgesic drugs to a patient as this will provide information on whether analgesic intervention is necessary.
For example, if the animal is very sedated from opioid administration it is possible timing of the next dose of opioid can be delayed.
Alternatively, if the animal is painful at the assessment it indicates more frequent analgesic administration is necessary and this should also be a trigger for more frequent pain assessment. If an animal is painful before drug administration it is also important to confirm an animal is comfortable following intervention (for example, drug administration should be followed by another pain assessment 30 minutes later).
Pharmacological management of postoperative pain
Opioids
Opioids form the mainstay of postoperative analgesia regimens because they are licensed, efficacious and can be given to effect.
Several new opioids have been licensed in dogs and cats so there are now a large number of different opioids to choose from for postoperative pain management. Predicting the “right” opioid for the individual patient can be challenging and should be based on the following factors:
- Requirement for analgesia.
Based on pain assessment and the procedure that has been carried out, a judgement needs to be made on whether the animal is likely to be experiencing mild, moderate or severe pain. - What other analgesic drugs have been administered
For example, if local anaesthetic techniques have been employed at the time of anaesthesia and surgery then it may be possible to administer an opioid of lower efficacy (such as buprenorphine rather than methadone). - Required duration of action
Generally, longer acting opioids such as buprenorphine or methadone (rather than pethidine or butorphanol) are preferred for the postoperative period to avoid the need for frequent redosing.
Fentanyl transdermal solution, licensed for administration to dogs, provides 96 hours of opioid analgesia after application and can be a good option for prolonged analgesia in selected cases. Fentanyl can also be administered parenterally by constant rate infusion (CRI) to provide analgesia for animals postoperatively that are experiencing moderate to severe pain.
A matrix for postoperative pain management with opioids is suggested in Table 3.
NSAIDs
Alongside opioids, NSAIDs are the other class of drug for which there is a large evidence base of efficacy for the management of postoperative pain (Slingsby and Waterman-Pearson, 2002; Leece et al, 2005; Dzikiti et al, 2006; Shih et al, 2008).
As well as forming the foundation of multimodal analgesia regimens in dogs and cats, they are also the go-to drug for postoperative pain management once the patient has been discharged from the clinic because they are efficacious, can be administered orally and are not subject to Controlled Drugs regulations.
There is a large number of NSAIDs licensed for postoperative administration to dogs, with a smaller repertoire of drugs in cats, which probably reflects the less predictable metabolism of NSAIDs in cats leading to a higher risk of side effects.
In recent years there has been a drive to develop NSAIDs that are more selective for the COX-2 enzyme, but no evidence supports the contention COX-2 selective drugs (for example, robenacoxib, firocoxib or cimicoxib) are safer than COX-2 preferential (for example, meloxicam or carprofen) NSAIDs (Hunt et al, 2014b).
In dogs, there is also no evidence to support greater analgesic efficacy of one NSAID over another, although it is noteworthy most NSAID studies are powered to only show equivalence between two NSAIDs rather than superiority of drug efficacy.
A study in cats (Kamata et al; 2012) compared postoperative analgesia for 24 hours after soft tissue surgery (mainly ovariohysterectomy) provided by a single dose of robenacoxib or meloxicam and found statistically significant differences between groups, with superior efficacy in the robenacoxib group for the primary efficacy end point of total clinician score. However, it is noteworthy all cats in the study were adequately analgesed, with no requirement for rescue analgesia in either treatment group, questioning the biological relevance of this finding.
The major disadvantage of NSAIDs is the risk of side effects in animals with concurrent disease, particularly in animals with renal disease or gastrointestinal injury (Monteiro-Steagall et al, 2013). Decision making about whether to administer an NSAID must be made on a case by case basis, but in contrast to chronic pain management, where licensed options for chronic pain relief are very limited, in the acute situation hospitalisation for the administration of systemic opioids or discharge with transdermal opioid solution can overcome the need for NSAID treatment.
Paracetamol (acetaminophen)
Paracetamol is very commonly administered for postoperative pain relief in humans, often in combination with NSAID treatment (Derry et al, 2013) without apparent adverse effects.
Although contraindicated in cats, there is a licensed formulation of paracetamol for dogs, which is a mixture of paracetamol and codeine. The codeine, however, is not bioavailable to dogs due to a high first pass metabolism of the opioid by the liver when absorbed via the portal system from the gut (Kukanich, 2010).
The drug is licensed for administration to dogs for a duration of five days only and has similar contraindications to NSAIDs with respect to concurrent liver, renal or gastrointestinal disease and is also contraindicated for coadministration with NSAIDs.
There is also an intravenous preparation of paracetamol being used in some veterinary hospitals, including the author’s own institution, for the management of postoperative pain in dogs. In people, the dose of IV paracetamol is recommended to be administered over 15 minutes, therefore slow infusion over a similar time period is also recommended in dogs. A dose rate of 10mg/ kg of parenteral paracetamol twice daily is the recommended dose rate for dogs, although there is no evidence base to support this dose.
There is an increasing tendency for paracetamol to be perceived as “safer” than NSAIDs, particularly with respect to adverse gastrointestinal events. The author advises caution because of a lack of data to support this contention and suggests paracetamol also be avoided in animals with gastrointestinal, renal or hepatic disease.
Analgesic adjuncts
Ketamine
Ketamine, an N-methyl-D-aspartate receptor antagonist, is being increasingly used IV by CRI to manage moderate to severe pain in cats and dogs, particularly where signs of central sensitisation are pronounced (such as in marked secondary hyperalgesia and allodynia).
Although there is very good evidence to support an antihyperalgesic effect of ketamine administered intrathecally in laboratory animal models (Burton et al, 1999; Boettger et al, 2010), the evidence base for the use of ketamine IV in cats and dogs is poor. A pivotal study in dogs (Wagner et al, 2002) showed ketamine administered at 10μg/kg/min intraoperatively (preceded by a 0.5mg/kg bolus) followed by 2μg/kg/min ketamine postoperatively had statistically lower pain scores in the postoperative period than dogs that did not receive ketamine, although all dogs received a fentanyl infusion for the first 18 hours after surgery.
However, the differences in pain score were marginal and it could be argued that although statistically significant the biological relevance of the difference in pain score was limited. The author recommends ketamine only be used as an adjunct to full μ agonist opioid analgesia and should not be regarded as a first line drug for the management of postoperative pain.
Lidocaine
A number of studies have investigated the intraoperative and postoperative analgesic effects of lidocaine IV given by CRI during anaesthesia (Ortega and Cruz, 2011; Columbano et al, 2012; Guttierrez-Blanco et al, 2013), but almost no studies document the analgesic effect of a lido caine CRI-administered during the postoperative period.
Guimarães Alves et al (2014) compared the postoperative analgesic effects of lidocaine (1mg/kg bolus slowly IV followed by a 50μg/ kg/min CRI), morphine (0.1 mg/kg/hour CRI) or morphine combined with lidocaine at the same dose rates in dogs undergoing fracture repair. In this study (Guimarães Alves et al, 2014) pain score and postoperative analgesic requirements did not differ between treatments, and pain scores were generally low in all dogs in all treatment groups, indicating most dogs were adequately analgesed by the test regimens.
Conversely, Gutierrez-Blanco et al (2013) investigated the postoperative analgesic effects of a lidocaine infusion (25μg/kg/min) and concluded analgesia was inadequate after ovariohysterectomy. Anecdotally, lidocaine is reported to be efficacious for the management of visceral-type pain, although there are no studies to support this contention. Similarly to ketamine, lidocaine is not recommended as a first line analgesic treatment in dogs and should only be used as an adjunct in combination with full μ agonist opioids.
No studies have investigated lidocaine for postoperative analgesia in cats. Apart from the probable greater risk of toxicity resulting from lidocaine CRI in cats compared to dogs, lidocaine during isoflurane anaesthesia is associated with significant cardiopulmonary depression (Pypendop and Ilkiw, 2005), although it is not known whether this degree of depression is also seen in conscious cats administered lidocaine. At the present time administration of a lidocaine CRI for cats for the provision of analgesia is not recommended.
Morphine-lidocaine-ketamine
The combination of morphine-lidocaine-ketamine has also been widely evaluated intraoperatively for the provision of balanced anaesthesia in dogs (Muir et al, 2003; Aguado et al, 2011; Ebner et al, 2013), but almost no studies have investigated this combination administered by CRI postoperatively for postoperative pain relief. The combination represents a multimodal approach to analgesia, which is recommended, but requires further evaluation before it can be routinely recommended for postoperative pain relief. The optimal dose rate of the three drugs used in combination in the postoperative period has also not been established.3 Morphine is also not licensed for use in dogs or cats; however, no published data describes the pharmacokinetics of a methadone CRI in either species.
Clinical approach to use of analgesic adjuncts
Given the lack of data it can be difficult to know what is the best approach to pain relief in animals experiencing severe pain that is poorly controlled by a full μ agonist opioid alone. The paradigm in Figure 2 is suggested.
These analgesic drugs are very potent, therefore it is recommended to give the drugs using a controlled infusion apparatus (such as a syringe driver) separately from each other so the effect of each drug can be individually assessed before further drugs are added (Figure 3). This also allows animals to be weaned off each drug in turn (starting with lidocaine, then ketamine, then the opioid CRI) as pain level declines over time.
Tramadol
Tramadol is very widely prescribed by veterinary practitioners for the management of postoperative pain (Hunt et al, 2014a), although the evidence base for the efficacy of oral tramadol for the management of postoperative pain in either cats or dogs is poor. Tramadol is a synthetic analgesic that has both opioid and non-opioid-like effects.
The parent drug is a racemic mixture of two complementary enantiomers of tramadol, + tramadol and – tramadol, which have effects to reduce the reuptake and increase the release of serotonin and inhibit the reuptake of noradrenaline and activate α2 receptors in the CNS. Collectively, serotonin and noradrenaline are important neurotransmitters in the descending control of analgesia and therefore modulation of serotonin and noradrenaline concentrations provides two mechanisms by which tramadol exerts an analgesic effect. The opioid-like effects of tramadol are mediated almost exclusively by an active metabolite produced by liver metabolism of tramadol, O-desmethyltramadol (M1).
Cats seem to generate reasonable concentrations of M1 after tramadol administration (Pypendop and Ilkiw, 2008) and may benefit from more opioid analgesia following tramadol administration than dogs, which probably generate much lower concentrations of M1 (Giorgi et al, 2010; Kukanich and Papich, 2011).
A few studies have investigated oral tramadol as a postoperative analgesia in dogs. Delgado et al (2014) compared oral tramadol (5mg/kg, two hours before premedication and 12 hours later) or oral carprofen at the licensed dose at the same time points in dogs undergoing enucleation. The requirement for rescue analgesia was significantly lower in the dogs that were treated with carprofen.
Davila et al (2013) compared pain scores following oral tramadol (4mg/kg to 5mg/kg every eight hours starting before surgery), firocoxib at the licensed dose or the combination of firocoxib and tramadol for three days after surgery in dogs undergoing tibial-plateau-levelling osteotomy. Dogs treated with tramadol alone had significantly higher pain scores and required more rescue analgesia than dogs in the firocoxib alone and firocoxib-tramadol groups.
These studies both suggest oral tramadol is less efficacious than an NSAID for postoperative pain relief in dogs and indicate that unless contraindicated in an individual patient, tramadol should not be used in preference to NSAID therapy. There also appears to be no benefit of combining tramadol and an NSAID together although more studies are required in this area.
No studies have investigated oral tramadol for postoperative pain relief in cats, although administration of tramadol to cats orally can be challenging due to its bitter taste, rendering it less practical for use in this species in the postoperative period.
Conclusion
Effective postoperative analgesia that prevents the development of upregulated pain states can only be achieved with good pre and intraoperative analgesia combined with regular pain assessment in the postoperative period.
Opioids and NSAIDs remain the mainstay of postoperative analgesia regimens and, for the majority of patients, judicious use of these classes of drugs alone will achieve effective analgesia. Adjuncts such as ketamine and lidocaine should be reserved for cases where pain management with traditional analgesics is inadequate and when used should ideally be administered via separate controlled infusion devices to allow the rate of each agent to be adjusted independently.
The evidence base for postoperative analgesia with oral tramadol is very poor, and tramadol should not routinely be used in preference to an NSAID in cats and dogs.
References
- Aguado D, Benito J and Gómez de Segura I A (2011). Reduction of the minimum alveolar concentration of isoflurane in dogs using a constant rate of infusion of lidocaine-ketamine in combination with either morphine or fentanyl, Vet J 189(1): 63-66.
- Balmer T V, Irvine D, Jones R S, Roberts M J, Slingsby L, Taylor P, Waterman A E and Waters C (1998). Comparison of carprofen and pethidine as postoperative analgesics in the cat, J Small Anim Pract 39(4):158-164.
- Boettger M K, Weber K, Gajda M, Bräuer R and Schaible H G (2010). Spinally applied ketamine or morphine attenuate peripheral inflammation and hyperalgesia in acute and chronic phases of experimental arthritis, Brain Behav Immun 24(3): 474-485.
- Brondani J T, Mama K R, Luna S P, Wright B D, Niyom S, Ambrosio J, Vogel P R and Padovani C R (2013). Validation of the English version of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in cats, BMC Vet Res 9:143.
- Brondani J T, Loureiro Luna S P, Beier S L, Minto B W and Padovani C R (2009). Analgesic efficacy of perioperative use of vedaprofen, tramadol or their combination in cats undergoing ovariohysterectomy, J Feline Med Surg 11(6): 420-429.
- Brondani J T, Luna S P and Padovani C R (2011). Refinement and initial validation of a multidimensional composite scale for use in assessing acute postoperative pain in cats, Am J Vet Res 72(2): 174-183
- Burton A W, Lee D H, Saab C and Chung J M (1999). Preemptive intrathecal ketamine injection produces a long-lasting decrease in neuropathic pain behaviors in a rat model, Reg Anesth Pain Med 24(3): 208-213.
- Columbano N, Secci F, Careddu G M, Sotgiu G, Rossi G and Driessen B (2012). Effects of lidocaine constant rate infusion on sevoflurane requirement, autonomic responses, and postoperative analgesia in dogs undergoing ovariectomy under opioid-based balanced anesthesia, Vet J 193(2): 448-455.
- Davila D, Keeshen T P, Evans R B and Conzemius M G (2013). Comparison of the analgesic efficacy of perioperative firocoxib and tramadol administration in dogs undergoing tibial plateau leveling osteotomy, J Am Vet Med Assoc 243(2): 225-231.
- Delgado C, Bentley E, Hetzel S and Smith L J (2014). Comparison of carprofen and tramadol for postoperative analgesia in dogs undergoing enucleation, J Am Vet Med Assoc 245(12): 1,375-1,381.
- Derry C J, Derry S and Moore R A (2013). Single dose oral ibuprofen plus paracetamol (acetaminophen) for acute postoperative pain, Cochrane Database Syst Rev 6: CD010210.
- Dzikiti T B, Joubert K E, Venter L J and Dzikiti L N (2006). Comparison of morphine and carprofen administered alone or in combination for analgesia in dogs undergoing ovariohysterectomy, J S Afr Vet Assoc 77(3): 120-126.
- Ebner L S, Lerche P, Bednarski R M and Hubbell J A (2013). Effect of dexmedetomidine, morphine-lidocaine-ketamine, and dexmedetomidine-morphine-lidocaine-ketamine constant rate infusions on the minimum alveolar concentration of isoflurane and bispectral index in dogs, Am J Vet Res 74(7): 963-970.
- Fox S M, Mellor D J, Stafford K J, Lowoko C R and Hodge H (2000). The effects of ovariohysterectomy plus different combinations of halothane anaesthesia and butorphanol analgesia on behaviour in the bitch, Res Vet Sci 68(3): 265-274.
- Giordano T, Steagall P V, Ferreira T H, Minto B W, de Sá Lorena S E, Brondani J and Luna S P (2010). Postoperative analgesic effects of intravenous, intramuscular, subcutaneous or oral transmucosal buprenorphine administered to cats undergoing ovariohysterectomy, Vet Anaesth Analg 37(4): 357-366.
- Giorgi M, Del Carlo S, Łebkowska- Wieruszewska B, Kowalski C J, Saccomanni G (2010). Pharmacokinetics of tramadol and metabolites after injective administrations in dogs, Pol J Vet Sci 13(4): 639-644.
- Guimarães Alves I P, Montoro Nicácio G, Diniz M S, Alves Rocha T L, Prada Kanashiro G and Navarro Cassu R (2014). Analgesic comparison of systemic lidocaine, morphine or lidocaine plus morphine infusion in dogs undergoing fracture repair, Acta Cir Bras 29(4): 245-251.
- Gutierrez-Blanco E, Victoria-Mora J M, Ibancovichi-Camarillo J A, Sauri-Arceo C H, Bolio-González M E, Acevedo-Arcique C M, Marin- Cano G and Steagall P V (2013). Evaluation of the isoflurane-sparing effects of fentanyl, lidocaine, ketamine, dexmedetomidine, or the combination lidocaine-ketamine-dexmedetomidine during ovariohysterectomy in dogs, Vet Anaesth Analg 40(6): 599-609.
- Holton L, Reid J, Scott E M, Pawson P and Nolan A (2001). Development of a behaviour-based scale to measure acute pain in dogs, Vet Rec 148(17): 525-531.
- Hunt J, Knowles T, Lascelles B D X and Murrell J (2014a). Prescription of perioperative analgesics by UK small animal veterinary surgeons in 2013, Proceedings of the Spring Meeting of the Association of Veterinary Anaesthetists, Nottingham, UK.
- Hunt J, Dean R and Murrell J (2014b). An analysis of the frequency of reported adverse events associated with NSAID administration in dogs and cats, Proceedings of the Spring Meeting of the Association of Veterinary Anaesthetists, Nottingham, UK.
- Kamata M, King J N, Seewald W, Sakakibara N, Yamashita K and Nishimura R (2012). Comparison of injectable robenacoxib versus meloxicam for peri-operative use in cats: results of a randomised clinical trial, Vet J 193(1):114-118.
- Katz J, Clarke H and Seltzer Z (2011). Preventive analgesia: quo vadimus? Anesth Analg 113(5): 1,242-1,253.
- Kukanich B and Papich M G (2011). Pharmacokinetics and antinociceptive effects of oral tramadol hydrochloride administration in greyhounds, Am J Vet Res 72(2): 256-262.
- KuKanich B (2010). Pharmacokinetics of acetaminophen, codeine, and the codeine metabolites morphine and codeine-6-glucuronide in healthy greyhound dogs, J Vet Pharmacol Ther 33(1): 15-21.
- Leece E A, Brearley J C and Harding E F (2005). Comparison of carprofen and meloxicam for 72 hours following ovariohysterectomy in dogs, Vet Anaesth Analg 32(4): 184-192.
- Linton D D, Wilson M G, Newbound G C, Freise K J and Clark T P (2012). The effectiveness of a long-acting transdermal fentanyl solution compared to buprenorphine for the control of postoperative pain in dogs in a randomized, multicentered clinical study, J Vet Pharmacol Ther 35(Suppl 2): 53-64.
- Martinez S A, Wilson M G, Linton D D, Newbound G C, Freise K J, Lin T L and Clark T P (2014). The safety and effectiveness of a long-acting transdermal fentanyl solution compared with oxymorphone for the control of postoperative pain in dogs: a randomized, multicentered clinical study, J Vet Pharmacol Ther 37(4): 394-405.
- Monteiro-Steagall B P, Steagall P V and Lascelles B D (2013). Systematic review of nonsteroidal anti-inflammatory drug-induced adverse effects in dogs, J Vet Intern Med 27(5): 1,011-1,019.
- Morgaz J, Muñoz-Rascón P, Serrano-Rodríguez J M, Navarrete R, Domínguez J M, Fernández-Sarmiento J A, Gómez-Villamandos R J, Serrano J M and Granados Mdel M (2014). Effectiveness of pre-peritoneal continuous wound infusion with lidocaine for pain control following ovariohysterectomy in dogs, Vet J 202(3): 522-526.
- Morton C M, Reid J, Scott E M, Holton L L and Nolan A M (2005). Application of a scaling model to establish and validate an interval level pain scale for assessment of acute pain in dogs, Am J Vet Res 66(12): 2,154-2,166.
- Muir W W 3rd, Wiese A J and March P A (2003). Effects of morphine, lidocaine, ketamine, and morphine-lidocaine-ketamine drug combination on minimum alveolar concentration in dogs anesthetized with isoflurane, Am J Vet Res 64(9): 1,155-1,160.
- Murrell J C, Psatha E P, Scott E M, Reid J and Hellebrekers L J (2008). Application of a modified form of the Glasgow pain scale in a veterinary teaching centre in the Netherlands, Vet Rec 162(13): 403-408.
- Ortega M and Cruz I (2011). Evaluation of a constant rate infusion of lidocaine for balanced anesthesia in dogs undergoing surgery, Can Vet J 52(8): 856-860.
- Pypendop B H and Ilkiw J E (2005). Assessment of the hemodynamic effects of lidocaine administered IV in isoflurane-anesthetized cats, Am J Vet Res 66(4): 661-668.
- Pypendop B H and Ilkiw J E (2008). Pharmacokinetics of tramadol, and its metabolite O-desmethyl-tramadol, in cats, J Vet Pharmacol Ther 31(1): 52-59.
- Robertson S A, Taylor P M, Lascelles B D and Dixon M J (2003). Changes in thermal threshold response in eight cats after administration of buprenorphine, butorphanol and morphine, Vet Rec 153(15): 462-465.
- Sano T, Nishimura R, Kanazawa H, Igarashi E, Nagata Y, Mochizuki M and Sasaki N (2006). Pharmacokinetics of fentanyl after single intravenous injection and constant rate infusion in dogs, Vet Anaesth Analg 33(4): 266-273.
- Shih A C, Robertson S, Isaza N, Pablo L and Davies W (2008). Comparison between analgesic effects of buprenorphine, carprofen, and buprenorphine with carprofen for canine ovariohysterectomy, Vet Anaesth Analg 35(1): 69-79.
- Slingsby L S, Murrell J C, Taylor P M (2010). Combination of dexmedetomidine with buprenorphine enhances the antinociceptive effect to a thermal stimulus in the cat compared with either agent alone, Vet Anaesth Analg 37(2): 162-170.
- Slingsby L S and Waterman-Pearson A E (2002). Comparison between meloxicam and carprofen for postoperative analgesia after feline ovariohysterectomy, J Small Anim Pract 43(7): 286-289.
- Slingsby L S and Waterman-Pearson A E (2001). Analgesic effects in dogs of carprofen and pethidine together compared with the effects of either drug alone, Vet Rec 148(14): 441-444.
- Wagner A E, Walton J A, Hellyer P W, Gaynor J S and Mama K R (2002). Use of low doses of ketamine administered by constant rate infusion as an adjunct for postoperative analgesia in dogs, J Am Vet Med Assoc 221(1): 72-75.
Figure 1. Careful assessment of pain, including wound palpation, is important in the postoperative period.
Figure 2. Paradigm illustrates an approach to pain relief poorly controlled by mu agonist opioid alone.
Figure 3. Analgesic adjuncts given by CRI should ideally be administered using separate controlled infusion apparatus.
Table 1. Advantages and limitations of the Glasgow Composite Pain Scale for dogs
Table 2. Advantages and limitations of the Botucatu Multidimensional Composite Pain Scale for cats
Table 3. Recommendations for the use of different opioids in the postoperative period
Latest news

Podcast
Vet Times Extra: Researching human-animal bond, with Tammie King and Vanessa Ashall
Sponsored
30 Apr 2025