3 Apr 2017
Allergen-specific immunotherapy in small animal patients – part 1
Debbie Gow and Hilary Jackson discuss modifying animals’ immune response to allergens to treat their skin disease, in the first of a two-part article.

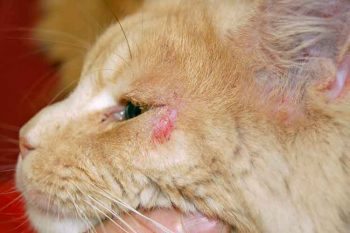
Figure 2. Atopic dermatitis with facial pruritus in a cat.
Canine atopic dermatitis (AD) is a common chronic disease that can account for up to 24% of non-routine cases presented to vets in the UK.
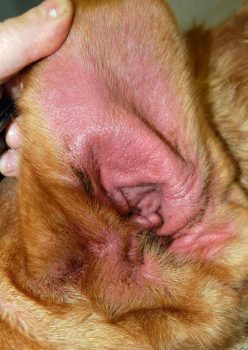
It is defined as a genetically predisposed inflammatory and pruritic skin disease, associated with the production of IgE antibodies, and most commonly directed against environmental allergens (Halliwell, 2006). Treatment and management of dogs can be a challenge for owners and vets, with therapy often tailored to the patient and involving multimodal therapy to control multiple aspects of the disease. Both topical and systemic anti-inflammatory and antimicrobial therapies are often used to reduce inflammation and control secondary infections. Therapeutic recommendations are based on evidence of efficacy (Olivry et al, 2015).
Following a diagnosis of AD, intradermal testing (IDT) or allergen-specific IgE serology is used to identify the environmental allergen(s) that initiate the disease. Allergen-specific immunotherapy (ASIT) can be used to modify the immune response to these allergens, with a long-term goal of reducing the requirement for additional treatment with immunosuppressive, anti-inflammatory drugs or antimicrobials.
Injectable ASIT has been used for many years to successfully treat allergic disease in human and veterinary patients. The application of sublingual immunotherapy (SLIT) in veterinary medicine has provided an additional method of immunotherapy delivery for dogs with AD. The use of SLIT as an alternative to injectable ASIT will be discussed in part two of this article. For the purpose of this part, ASIT specifically refers to the use of SC immunotherapy.
AD pathogenesis
Canine AD is a complex disease that involves immune dysregulation, allergic sensitisation, skin barrier defects, microbial colonisation and environmental factors (Nuttall et al, 2013). Both circulating and cutaneous lymphocyte populations (T and B lymphocytes) play important roles in the development and progression of AD. Initially, inflammation and the cytokines produced stimulate the production of a subtype of T lymphocyte, T-helper 2 cells (Th2). These cells stimulate the production of allergen-specific IgE, induce cytokine-mediated inflammation (interleukin 4) and also activate cells associated with hypersensitivity (eosinophils).
This response is associated with the acute stage of disease, while another type of T lymphocyte (Th1 cells) is associated with chronic disease. An animal is sensitised to an allergen following antigen (allergen) capture and processing by an antigen-presenting cell (APC). APCs present the antigen to Th2 lymphocytes, thereby stimulating IgE production from B lymphocytes. The allergen-specific IgE then binds to receptors on the surface of mast cells. Repeated exposure to the same allergen results in degranulation of mast cells releasing histamine and pro-inflammatory mediators (Figure 100).
Immunotherapy mechanism
The aim of immunotherapy is to administer increasing doses of known allergens, to which an animal is hypersensitive, to modify the immune system. This results in a switching of the immune response from over-reactive to tolerance. The exact mechanism of action of ASIT in human and canine AD is still not fully understood, although it is known ASIT alters many aspects of the immune response, including antibody production, cytokine production and T cell activation. This is mainly achieved by reduction of allergen-specific IgE, upregulation of T-regulatory cells and Th1 cells to suppress immune function.
Regulatory T cells are known to secrete anti-inflammatory cytokines interleukin 10 and transforming growth factor beta, both of which are reduced in atopic dogs and increased in dogs successfully managed with ASIT (Keppel et al, 2008). In addition, ASIT will also increase the production of blocking IgG antibodies that compete with allergen-specific IgE (Fraser et al, 2004; Hites et al, 1989). For successful immunotherapy, there must be not only an increase in the T cell population, but, specifically, a shift to the Th1 cell population (Shida et al, 2004).
ASIT in human and vet medicine
Treatment of human allergic rhinitis using ASIT was first described more than a century ago and, since then, it has been used for many human conditions, including conjunctivitis, asthma and arthropod bite hypersensitivity. The use of this therapy in dogs was first reported more than 70 years ago (Griffin and Hillier, 2001). Many reports and studies have subsequently described the safe and effective use of this disease-modifying therapy in atopic dermatitis in dogs, cats and horses (Griffin and Hillier, 2001; Loewenstein and Mueller, 2009).
It is also the only therapy that can modify the natural course of a pre-existing hypersensitivity (Griffin and Hillier, 2001). A survey showed one-third of owners of atopic dogs rated ASIT, when used as part of therapy, as very, or extremely, effective long-term therapy (5 to 10 years), with 5% of dogs achieving complete remission (Dell et al, 2012).
Patient selection
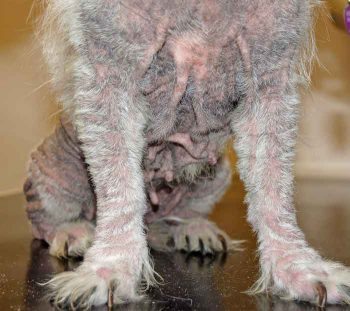
Immunotherapy should be offered to any dog or cat where AD has been diagnosed, and the likely allergens causing disease have been identified (IDT or IgE serology). It is generally most beneficial for patients with non-seasonal allergies, although this does not exclude its use in cases of seasonal AD. Patients selected for immunotherapy should ideally be diagnosed at a young age, with the aim of using immunotherapy to help prevent the development of further allergy and before chronic changes develop, which may complicate the management of such cases (Figure 3).
Immunotherapy should also be used in animals that have not responded to systemic anti-inflammatory therapeutics, have undesirable side effects associated with these, or in patients where long-term use of such drugs would not be ideal. Additional factors to consider should be the owner’s compliance, finance, time and acceptability to deliver immunotherapy at home.
Allergen selection
ASIT should be tailored for the individual animal. Allergens to include in an ASIT protocol are selected based on either IDT or IgE serology, or a combination of both (Figure 4). Considering serological testing, a great deal of variability can occur in the results obtained from different laboratories. This is presumably due to different allergen source material, detecting antibodies and cut-off values employed in the individual laboratory’s serological assays. Immunotherapy recommendations based on serum allergy test results will also be varied as a result (Plant et al, 2014).
The intradermal test measures mast cell-bound allergen-specific IgE and the response of the mast cell itself after allergen capture by antibody. IgE in the skin is not necessarily directly proportional to serum IgE concentrations. It is also important to note healthy dogs may also have positive results from IgE serology, or immediate positive reactions using IDT to environmental allergens.
With the aforementioned in mind, it is important selection of allergens should not be based on these test results alone, but the clinical history, seasonality of the clinical signs and geographical location must all be considered, and allergens considered to be “clinically relevant” selected.
Concurrent therapy and control of flare factors
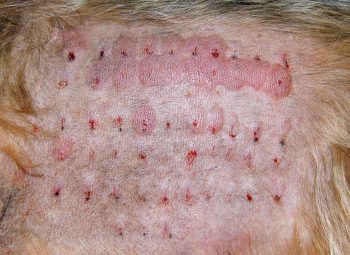
In the initial stages of immunotherapy, anti-inflammatory medication is often still required as allergen-specific immune-modulation may take several months (sometimes up to a year) to achieve. ASIT may be combined safely with one or more of antihistamines, glucocorticoids, ciclosporin, essential fatty acids, oclacitinib, antimicrobial therapy and topical preparations. At the time of writing, no studies are performed in animals to determine whether any of these treatments affect the outcome of ASIT. Readers are directed to the 2015 International Committee on Allergic Disease of Animals treatment guidelines for canine AD for further information on the use of these drugs (Olivry et al, 2015).
Flare factors that will cause an exacerbation of clinical signs must also be controlled or eliminated. These include the treatment or prevention of ectoparasites, controlling secondary infections (bacterial and/or fungal), reducing stress and determining if food is also contributing to clinical disease. Allergen avoidance, if possible, may be beneficial.
It is useful to educate clients in terms of their animals’ specific hypersensitivities with regard to putting any avoidance measures in place – for example, keeping their pet inside during episodes of high pollen counts, avoiding areas with high allergen concentrations (such as grassland if grass pollen is a known allergen) and minimising house dust mite exposure in the house. However, it must be remembered allergen avoidance in isolation is unlikely to achieve and maintain control of clinical signs associated with AD.
Adverse effects
Immunotherapy has a good record of safety in both human and veterinary medicine, with severe reactions being very rare (Griffin and Hillier, 2001). The most common side effect noted during immunotherapy is increased pruritus after injection. This can last up to two days, therefore a short course of glucocorticoids at this time may be indicated, starting the day before the injection is given. Local injection site inflammatory reactions have been reported, but do not generally require therapy. Vomiting within one hour after ASIT may also occur. Some dogs may also experience a reduced appetite for two to three days. The overall rate of systemic reactions in dogs (weakness, depression, anxiety, diarrhoea, vomiting, collapse and death) is reported at 1% (Baumer et al, 2011; Loewenstein and Mueller, 2009).
Factors potentially affecting treatment outcome
The main factors that will affect the outcome of immunotherapy are the correct selection of allergens to include in the therapy and the correct concentration. Other factors – such as age of disease onset, age at commencement of ASIT, duration of disease, severity of disease, strength of IDT result and number of IDT-positive results – have not been shown to adversely affect the outcome. The decision to start a patient on immunotherapy should therefore not be based on these observations.
Apparent immunotherapy failures should be re-evaluated with further IDT to determine if the pattern of sensitivity has changed over time. This is especially important for animals started on ASIT at an early age. Repeat IDT or serological testing should also be performed if apparent ASIT failure is experienced with a change of geographical location to an area with a different environmental allergen profile. Correct selection of allergens to include may be difficult to select using IgE serology alone due to circulating IgE not necessarily correlating with clinical disease, but demonstrating exposure.
Expected treatment outcome with immunotherapy
An evaluation of the clinical benefit of ASIT should not be made until a full year of treatment has been achieved. In most cases, immunotherapy will need to be continued for the rest of the animal’s life; however, reduction in the injection frequency and dose can be attempted in cases that are stable with complete remission of clinical signs. ASIT may also be stopped in these cases if deemed appropriate. Apparent ASIT failures may benefit from receiving SLIT instead of injectable ASIT (see part two).
A successful treatment outcome would be a reduction in the severity of the patient’s clinical signs associated with AD. This may be one or more of a reduction in pruritus, inflammation, the number of secondary infections or an improvement in the quality of life. Reported success rates with ASIT range from 50% to 100%; however, variation in allergen dose, type, concentration and response criteria make these results difficult to interpret. In a double-blind randomised study, a 50% improvement in pruritus was observed in 45% to 55% of dogs (Mueller et al, 2005).
While immunotherapy alone is unlikely to cure AD, it can help prevent disease progression and reduce the dependency of the animal on anti-inflammatory drugs that have systemic effects, such as glucocorticoids.
Immunotherapy in feline patients
While the focus of this article is on the use of ASIT in canine patients, ASIT should also be considered as a safe therapeutic option for cats diagnosed with AD and allergic asthma. The reported success rate in cats is 50% to 75% (Trimmer et al, 2006). The use of ASIT in cats offers the same advantages as with the dog, although owners may be less likely to administer the injection at home, depending on the temperament of the cat. SLIT may be a useful alternative in these patients (see part two). Conversely, ASIT may be beneficial for owners that cannot administer oral medication.
Conclusion
ASIT has been used for many years to successfully treat human and veterinary patients with AD. It should always be considered and offered to clients with dogs and cats diagnosed with AD associated with exposure to environmental allergen(s) as an effective long-term treatment. Clients must be informed clinical improvement is not seen in every patient. The time for a clinically useful effect to be noted can be up to 12 months, therefore additional therapy is often indicated to achieve clinical improvement and a suitable quality of life for the dog.
Variation in allergen identification, selection and formulation (concentration, dose and number of allergens) make comparing the effect of different protocols difficult.
Further research is needed using ASIT in dogs with AD to determine the true beneficial effects of such therapy and standardise allergen selection and delivery. In addition, human trials using alternative routes of delivery, such as epicutaneous and intralymphatic, have produced exciting results, with preliminary studies in dogs underway (DeBoer, 2017). This is an area that will hopefully develop over the next few years to allow us to deliver the best treatment to companion animals with AD.
