6 Jun 2016
Approaches to treating a dog with supraventricular tachyarrhythmia

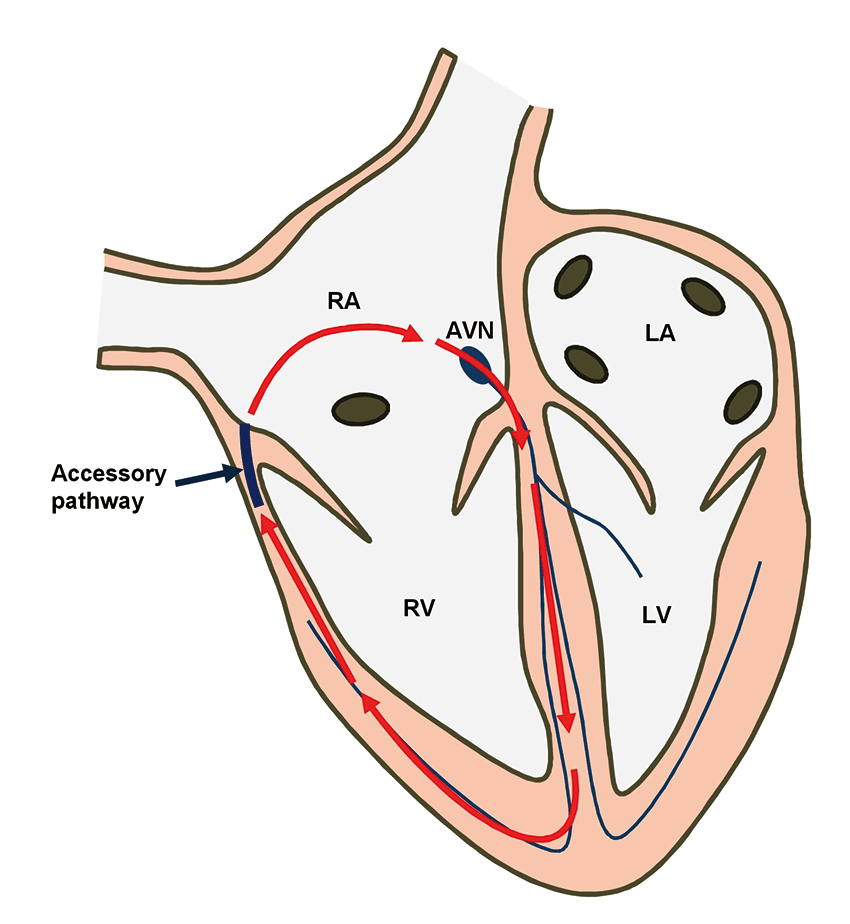
Figure 3a. Position of the accessory pathway.
Cardiac arrhythmias that lead to excessively high heart rates may be very debilitating and even result in life-threatening situations.
They are most commonly seen in older patients that suffer from structural heart disease, such as valvular degeneration or cardiomyopathies. Less commonly, younger patients may also be diagnosed with a tachyarrhythmia – particularly those with severe structural disease due to congenital heart defects (for example, patent ductus arteriosus).
In this article, the case of a young dog with a particular type of tachyarrhythmia, due to a congenital abnormality, is described. The name of this arrhythmia is orthodromic atrioventricular reciprocating tachycardia (OAVRT) and it is a type of supraventricular tachycardia (SVT).
Perhaps the name used in human medicine, Wolff-Parkinson-White syndrome, will be more familiar to most readers. It is due to the presence of an abnormal (accessory) conduction pathway between the atria and the ventricles that allows a macroreentrant circuit to occur – leading to tachycardia (Figure 1). These patients suffer from sustained periods of excessively fast heart rate that lead to signs of weakness, collapse and even myocardial failure in the form of tachycardia-induced cardiomyopathy (TICM).
This form of cardiomyopathy looks similar to dilated cardiomyopathy (DCM) on echocardiography, which may lead to a misdiagnosis. It is important to remember DCM is not common in young dogs and other possible causes should always be investigated.
The electrocardiographic diagnosis of OAVRT can also be quite challenging and, for this reason, it is likely many patients with this type of problem do not receive a correct and timely diagnosis. Death or euthanasia is a common outcome and there is also a lack of awareness from veterinary clinicians to the existence of these conditions.
The mainstay of treatment for these patients is to control the heart rate, which is normally attempted with anti-arrhythmic drugs. If successful, most dogs are able to recover fully from TICM. However, another option exists that is far more effective and has become the norm in human medicine. It is possible to locate the abnormal pathway inside the heart and destroy it by performing an electrophysiology study and radio catheter ablation. A cure is achieved in more than 95% of cases without the need for further treatment and this was the treatment chosen for this patient.
Signalment and history
When aged 11 months, a male Labrador retriever was presented to Southern Counties Veterinary Specialists with lethargy, inappetence, depressed demeanour and cough signs. At one year nine months, it was re-presented as weak and lethargic. It’s heart rate was approximately 300bpm and pulse quality was weak.
Diagnostic procedure
An electrocardiogram revealed the presence of periods of SVT, with a heart rate of more than 300bpm (Figure 2a). Small, negative deflections were noticed at the level of the ST segment suggesting retro-conducted P waves occurring shortly after the QRS complex. Additionally, during periods of sinus rhythm, the presence of beats with a short PR interval and a small positive deflection (delta wave) were noticed, suggesting pre-excitation of the ventricles via an accessory pathway (Figure 2b). These findings were suggestive of OAVRT.
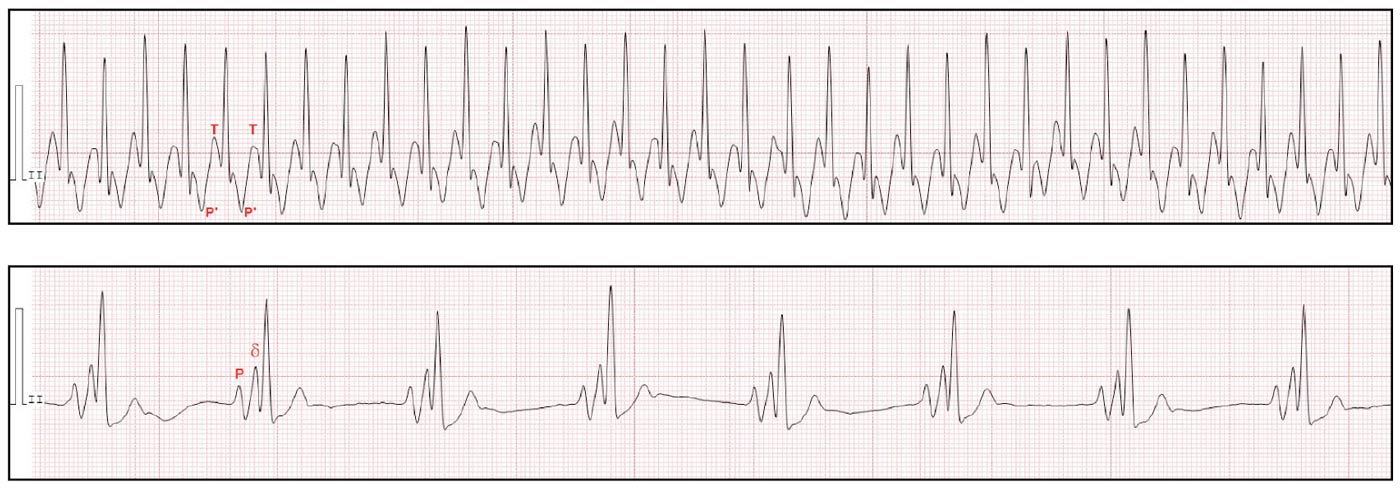
Figure 2b. Sinus rhythm. The PR interval is short and a small positive deflection is seen between the P wave and QRS (δ wave) suggesting pre-excitation via an accessory pathway.
An echocardiographic examination revealed cardiac enlargement with a reduction in systolic function signalling the presence of TICM. Elevated levels of cardiac troponin I suggested ongoing cardiomyocyte damage.
Chest radiographs revealed the presence of venous congestion and mild lung interstitial infiltrate consistent with pulmonary oedema.
Blood analysis did not show significant findings. ECG telemetry monitoring showed persistent SVT.
Treatment
With a bodyweight of 24.5kg, treatment was started with sotalol (40mg bid) and pimobendan (5mg bid), which initially seemed to control SVT and clinical signs; however, two weeks later it was presented again with refractory SVT. On this occasion, arrhythmia converted with intravenous lidocaine. Sotalol was stopped and diltiazem (90mg tid) and flecainide (50mg bid) were started. During the subsequent three days, Holter monitoring recorded one sustained episode of SVT, which lasted more than four hours, and occasional shorter episodes lasting from a few seconds up to five minutes. Mexiletine (200mg bid) was started.
A further Holter monitor recording showed a reduced number of SVT episodes, but sustained episodes were still present. There appeared to be an association with excitement and onset of SVT, so atenolol (12.5mg bid) was introduced and flecainide was stopped.
Tertiary referral for ablation therapy was discussed, but declined at the time.
A period of six weeks followed, during which time the dog was clinically normal. Echocardiographic examination showed normal systolic function and chamber dimensions had returned to normal. Holter examination showed better overall control of SVT, but sustained episodes were still present. Although SVT was not fully controlled on medical treatment, its frequency was significantly reduced to allow systolic function to recover. Pimobendan was stopped.
The dog then enjoyed a clinically stable period for the next seven months; however, the owner became aware of increasing SVT frequency (by palpating apex beats), so atenolol was switched for sotalol (20mg bid). Two months later, he presented with lethargy, cough, tachypnoea with refractory SVT and congestive heart failure. Pimobendan was restarted (7.5mg bid). SVT was initially controlled with an increased dose of sotalol (80mg bid), but lethargy signs quickly recurred and ECG confirmed refractory SVT.
All oral anti-arrhythmia therapies were stopped. SVT was converted with intravenous lidocaine and amiodarone (200mg bid, reduced to 200mg sid after seven days) was introduced. The dog appeared clinically well on amiodarone and pimobendan; however, repeat Holter monitoring showed SVT was present 65% to 70% of the time.
Ablation therapy was recommended again and it was referred to Davies Veterinary Specialists for treatment.
Electrophysiology study and radio catheter ablation.
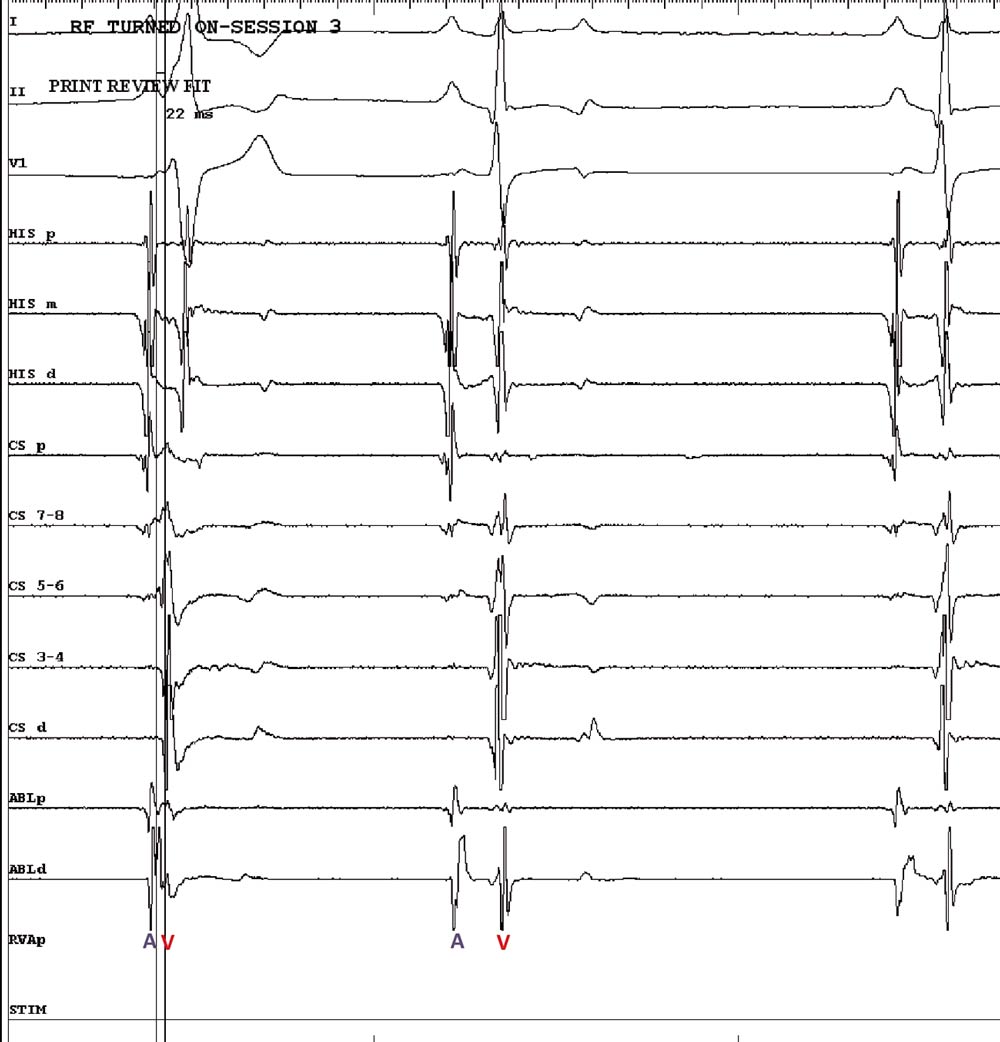
An electrophysiology study is a minimally invasive procedure in which catheters are positioned at specific sites in the heart to record intracardiac ECGs. Analysis of these ECGs during sinus rhythm and tachycardia allow the identification of the arrhythmia mechanism and the location of the abnormal area(s) in the heart from which the arrhythmia arises – in this case, the accessory pathway.
The procedure was performed under general anaesthesia, with the patient in dorsal recumbency. Two catheters were introduced into the heart via a jugular vein and another from a femoral vein under fluoroscopic guidance. After a series of tests, the accessory pathway was located on the right side of the heart over the tricuspid annulus in a caudolateral position (Figures 3a and 3b).
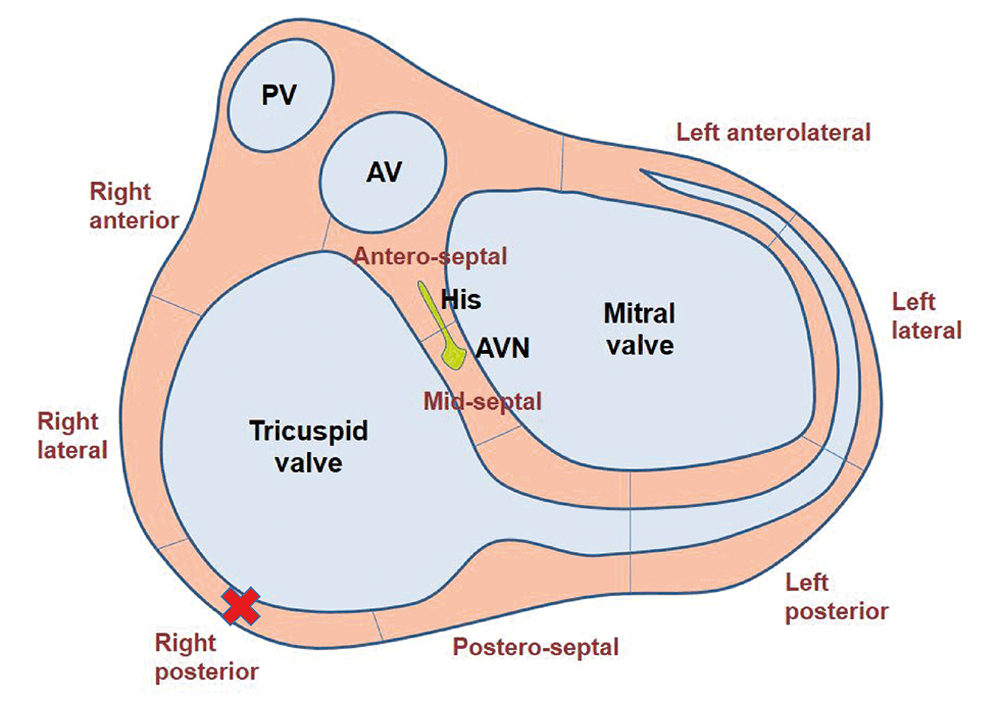
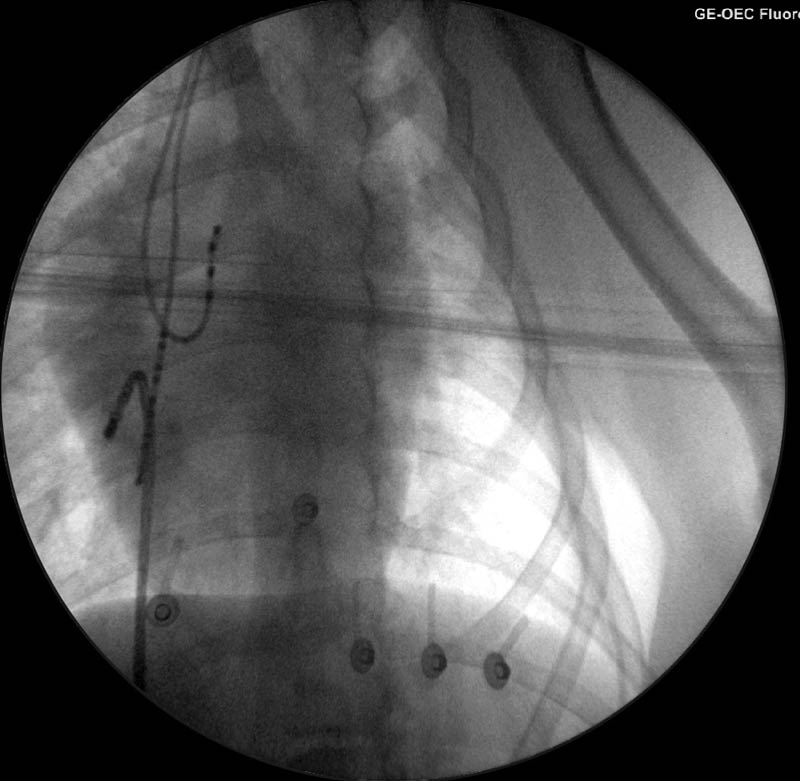
Radiofrequency energy was then delivered to this area with a special catheter to destroy the pathway and prevent further communication between the atrium and ventricle via this route – interrupting the arrhythmia mechanism (Figure 4). This was performed safely and with success. The entire procedure lasted approximately two hours.
Follow-up
The patient recovered well from anaesthesia without any complications. It was discharged the following day with a five-day course of meloxicam. All cardiac medications were stopped immediately and the dog has been doing well since without recurrence of the arrhythmia.
Concluding thoughts
This case is an example of a condition unknown to many veterinary clinicians and a rare example of a cardiac disease for which a cure is possible. The facilities to perform these sorts of procedures are rare in the veterinary world, but exist in this country and, hopefully, will become increasingly available in the future. They are very safe and effective. In humans they have become the first-line treatment for many cardiac arrhythmias instead of anti-arrhythmic drugs.
Atrial flutter, automatic atrial tachycardias, atrial fibrillation and some ventricular arrhythmias are examples of other arrhythmias that may be treated in this way.
- Please note some drugs in this article were used under the cascade.
