5 Sept 2023
Between a rock and a hard place – treating OA in cats
Karen Perry and Lauren Meneghetti explore the difficulties of handling these cases, such as drug interactions/complications and owners’ wishes.

Figure 3. Cataleyia, an 11-year-old, female, spayed domestic shorthair, presenting for intermittent shifting thoracic limb lameness of two months duration.
Treatment of feline OA is multimodal, including environmental and activity modulation, physical rehabilitation, dietary modulation and drug therapy, with analgesic treatment classically being based on the use of NSAIDs.
Although NSAIDs are undoubtedly effective in controlling joint pain and inflammation, safety concerns exist because of the high concurrent prevalence of other medical conditions in the clinical population of cats with OA. The importance of obtaining a complete patient history and considering the potential for medication interactions cannot be over-emphasised. Either the presence of a concomitant disorder, or the associated therapeutics, may necessitate a dose-reduction or, alternatively, render NSAID use inappropriate.
In this article, the authors will tackle two cases where NSAID use was either contraindicated or was not accepted by the owner. Alternative treatment options, including adjunctive analgesics, anti-nerve growth factor antibody, cognitive enrichment and weight loss, will be discussed based on a combination of peer-reviewed evidence and personal experience.
Keywords: cat, OA, medication interaction, clinical metrology instrument, multimodal analgesia
When contemplating common conditions that affect quality of life in cats, many will focus on ailments such as chronic kidney disease, hyperthyroidism, diabetes, hypertrophic cardiomyopathy and inflammatory bowel disease.
While these diseases occur commonly within the feline population, with an estimated prevalence of between 40% to 90% based on radiographic detection of joint changes, OA warrants a place on this list; in fact, some would argue that it should be at the top (Hardie et al, 2002; Clarke et al, 2005; Lascelles et al, 2010; Slingerland et al, 2011; Lascelles et al, 2012; Kimura et al, 2020).
While the divergence between the prevalence of radiographic changes (40% to 90%) and the number of cats being definitively diagnosed with osteoarthritis (1.4% to 2%; O’Neill et al, 2014; O’Neill et al, 2023) can partly be explained by the well-recognised discrepancy between radiographic changes and detectable clinical signs, greater awareness would likely go a long way towards remedying this incongruity.
When managing OA-associated pain, NSAIDs represent a mainstay of treatment in both dogs and cats. Multiple studies have demonstrated their unquestionable efficacy for management of the pain and inflammation associated with feline OA, specifically (Clarke and Bennett, 2006; Lascelles et al, 2007; Guillot et al, 2013; Adrian et al, 2021).
One of the key aspects rendering NSAID therapy attractive is the growing evidence base regarding use of the drugs in cats, with all studies showing a consistent benefit of their use.
This is in stark contrast to the limited evidence base supporting adjunctive analgesics, where results are less convincing (Vettorato and Corletto, 2011; Barbeau-Grégoire et al, 2022; Cunningham et al, 2022), partly due to the high incidence of the placebo effect that appears to be present in many studies (Vettorato and Corletto, 2011; Gruen et al, 2017; Shipley et al, 2021; Cunningham et al, 2022).
Although NSAIDs are undoubtedly effective in controlling joint pain and inflammation, safety concerns exist because of the high concurrent prevalence of other medical conditions in the clinical population of cats with OA. A distinct paucity of data exists regarding the safety of NSAIDs in populations with concomitant conditions, and some comorbidities may either necessitate a dose-reduction or, alternatively, render NSAID use inappropriate.
Additionally, NSAIDs are not always sufficiently effective when used as a monotherapy. Increasingly, we have to consider what options we have for OA treatment in cats when NSAIDs are contraindicated.
The following cases will address scenarios where NSAID use was either considered inapposite or was not accepted by the owner. Alternative treatment options will be discussed based on a combination of peer-reviewed evidence, anecdotal recommendations and personal experience.

Case one: Simon
Simon, a seven-year-old, male, neutered domestic shorthair, presented for an annual check-up (Figure 1).
The owner reported no concerns, but during a routine questionnaire performed at the hospital for all feline patients at every annual exam (Enomoto et al, 2020), it was noted that during the past year, Simon seemed to be playing less frequently with his toys and was jumping up on the bed less often when compared to answers given one year prior.
He was otherwise eating and drinking normally, with no vomiting, diarrhoea, coughing or sneezing. He had a history of recurrent urinary obstructions and pica, which was previously worked-up by a veterinary behaviourist, and both ailments appeared to be related to instances of stress and anxiety. He was prescribed fluoxetine two years prior and had not had any bouts of foreign body ingestion or urinary signs since. He was not taking any additional medications.
Simon’s general physical examination was unremarkable, but secondary to concern for chronic pain due to the answers given on the Feline Musculoskeletal Pain Screening Checklist (Enomoto et al, 2020), an orthopaedic exam was also performed, and a full Feline Musculoskeletal Pain Index (FMPI) completed by the owner (Enomoto et al, 2022). Simon was a nervous cat in the hospital and refused to walk around the room, so a thorough gait analysis was not performed.
Simon did, however, tolerate a full orthopaedic exam and was noted to be very stoic during this process. Simon was resistant to full extension of both of his coxofemoral joints, but the remainder of his examination was unremarkable. Pelvic radiographs were performed along with a full routine blood work panel (complete blood cell count [CBC] and serum chemistry) and urinalysis (UA).
The serum chemistry was unremarkable, except a mild elevation in total protein, suspected to be secondary to mild dehydration. The CBC showed no significant findings. The UA showed a normal urine specific gravity with no evidence of infection or inflammation.
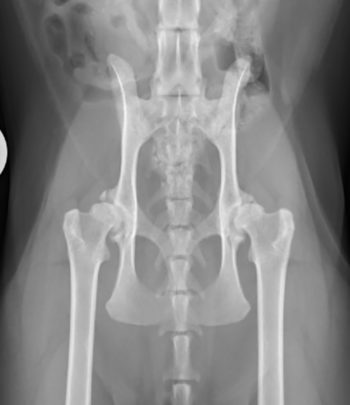
Pelvic radiographs revealed coxofemoral subluxation, osteophyte formation on the acetabular margins and both remodelling and subchondral sclerosis of the femoral head bilaterally (Figure 2). He was diagnosed with bilateral coxofemoral OA, likely secondary to initial hip dysplasia.
The results from the questionnaire, coupled with the orthopaedic examination and radiographic findings, indicate that Simon is experiencing OA-associated pain. At first glance, he might seem like an ideal candidate for NSAID use to manage this pain and address the associated mobility impairment. His blood work and urinalysis are within normal limits, and he has no known comorbidities that would contraindicate NSAID use.
However, we also have to consider the potential for drug interactions, and we know that Simon has been taking fluoxetine for two years to manage his anxiety-related behaviours. It has been shown that the combination of a serotonin reuptake inhibitor (SSRI), such as fluoxetine, and an NSAID increases the risk for gastrointestinal ulceration and bleeding (De Jong et al, 2003; Dalton et al, 2003; Tata et al, 2005; Helin-Salmivaara et al, 2007; Loke et al, 2008), just as we recognise with the combination of a steroid and an NSAID (Stanton and Bright, 1989; Dow et al, 1990; Kataoka et al, 2000; Hernández-Díaz and Rodríguez, 2001; Hinton et al, 2002; Boston et al, 2003; Mort et al, 2006; Narita et al, 2007).
The negative interaction between SSRIs and NSAIDs relates to platelet function. Serotonin is a necessary molecule in platelet activation and aggregation, and with SSRIs, reuptake of serotonin is blocked, resulting in impaired haemostatic function (Singh, 1998; Wolfe et al, 1999; Halperin and Reber, 2007). The negative effect on platelet function is synergised with concomitant NSAID use, due to the associated inhibition of cyclooxygenase enzymes, preventing formation of thromboxane A2, another key factor in platelet activation and aggregation (Hamberg et al, 1975; Rome and Lands, 1975; Stanford et al, 1977; Siess et al, 1985; Smith et al, 1985).
Tramadol has been shown to be a viable pain-relieving modality in cats (Monteiro et al, 2016; Monteiro et al, 2017; Guedes et al, 2018a), which is in stark contrast to similar studies performed in dogs (Budsberg et al, 2018).
While the mechanism of action of tramadol is not fully understood, tramadol has been reported as a μ-opioid receptor agonist, in addition to inhibiting nicotinic acetylcholine receptors, N-methyl-D-aspartate (NMDA) receptors, α2-adrenoceptors, and others (Desmeules et al, 1996; Frink et al, 1996; Minami et al, 2015).
The elevated efficacy in pain management in cats is suspected to be secondary to a high bioavailability, in addition to a longer half-life and higher concentrations of the active metabolite of tramadol, O-desmethyl-tramadol (M1; KuKanich and Papich, 2004; Pypendop et al, 2009). Studies focusing on the potential role for tramadol in the management of OA pain in cats report a reduction in central sensitisation, increased mobility and weight-bearing ability, and owner perception of improved quality of life (Monteiro et al, 2016; Monteiro et al, 2017; Guedes et al, 2018a).
Unfortunately for Simon, the current use of fluoxetine also renders the use of tramadol inappropriate. Tramadol also inhibits serotonin reuptake and, at high doses, primarily induces serotonin release (Reimann and Schneider, 1998). When combined with a SSRI, it increases the risk of serotonin syndrome (Egberts et al, 1997; Mason and Blackburn, 1997; Kesavan and Sobala, 1999; Lange-Asschenfeldt et al, 2002; Mahlberg et al, 2004; Mittino et al, 2004; Kitson and Carr, 2005; John and Koloth, 2007; Nayyar, 2009; Peacock and Wright, 2011; Shakoor et al, 2014).
Serotonin syndrome stems from elevated levels of serotonin within the brain, inducing neurological signs such as hyperactivity, seizures, ataxia, hyperthermia, and head weaving/shaking (Corne et al, 1963; Grahame-Smith, 1971). In addition, SSRIs such as fluoxetine are noted to be potent inhibitors of the hepatic enzyme CPY2D6 (Crewe et al, 1992; Jeppesen et al, 1996), a necessary enzyme in the metabolism of tramadol into its active metabolite, M1 (Paar et al, 1992). Inhibition of CPY2D6, therefore, decreases the pain-relieving efficacy of tramadol (Frost et al, 2019).
Gabapentin is a widely used medication for management of chronic pain in cats due to its safety and lack of major side effects. However, peer-reviewed literature documenting efficacy for management of OA-associated pain, or even pain associated with other chronic feline conditions, is sparse. Isolated case reports note potential benefit as an adjunctive analgesic for use in musculoskeletal pain and head trauma (Lorenz et al, 2013), and after traumatic events (Vettorato and Corletto, 2011). In the only known study evaluating gabapentin as a sole analgesic for management of OA-associated pain in cats, results indicated a significant improvement in impaired activities for cats receiving gabapentin when compared to those receiving placebo treatment, and that cessation of gabapentin resulted in a recurrence of that impairment (Guedes et al, 2018b).
However, adverse effects were noted in half of the cats included in this study, including sedation, pelvic limb weakness/ataxia, muscle tremors and diarrhoea.
Amantadine, which was originally used as an antiviral medication in humans, has been recognised as an antagonist of NMDA receptors. NMDA receptor activity plays a central role in the aetiology of central sensitisation and chronic pain (Bennett, 2000), which are recognised contributors to OA-associated pain in cats (Guillot et al, 2014; Guillot et al, 2015).
A blinded, placebo-controlled study evaluated the use of amantadine on quality of life and mobility in osteoarthritic cats, and demonstrated that amantadine was associated with significant improvement in mobility-impaired cats as assessed by their owners (Shipley et al, 2021). In the same study, lower activity counts during amantadine treatment were noted in comparison to cats receiving placebo treatment and this was postulated to be due to a mild sedative effect of amantadine.
No other side effects or drug interactions have been reported in cats receiving amantadine, and both are also rare in humans (Aoki and Sitar, 1988).
Elimination of amantadine is primarily via renal clearance by both glomerular filtration and tubular secretion; as such, amantadine accumulates in the plasma in patients with renal impairment, and dose adjustments should be considered in such cases (Aoki and Sitar, 1988).
In humans, amantadine does also lower the seizure threshold (Dragaševic-Miškovic et al, 2019); therefore, use should be carefully considered in any cat with a history of seizure activity.
A relatively novel therapeutic option for the management of OA-associated pain in cats is anti-nerve growth factor antibody. Nerve growth factor is a molecule involved in the pathway for transmission of a painful stimulus to the brain; it has also been shown to promote inflammation (Mantyh et al, 2011; Bannwarth and Kostine, 2015).
Anti-nerve growth factor antibody binds to nerve growth factor and prevents the inciting pathway leading to a pain stimulus. Results from studies investigating the use of anti-nerve growth factor antibody in cats suffering from osteoarthritic pain appear to be promising, with owner-assessed improvement evident for up to eight weeks and vet-assessed improvement persisting for up to 12 weeks (Gruen et al, 2015; Gearing et al, 2016; Gruen et al, 2016; Gruen et al, 2021a; Gruen et al, 2021b). Adverse events, when they occur, appear to be relatively mild, and do not differ in frequency when compared to a placebo-treated group, with the exception of episodes of skin irritation (Gruen et al, 2021a).
Following a discussion of the options, Simon’s owner elected to trial monthly SC injections of anti-nerve growth factor antibody, as currently recommended (Gruen et al, 2021a; Gruen et al, 2021b). The ability to avoid administering more medications at home, which was not always easy with the current fluoxetine, rendered this an attractive option for this particular owner.
Re-examination was planned for in one month, with a repeated FMPI (Enomoto et al, 2022) and orthopaedic examination to assess the therapeutic efficacy of the injections based on changes in scores and reported findings. Simon showed a significant improvement with the use of anti-nerve growth factor alone, but should additional therapeutics have been warranted, the inclusion of amantadine and/or gabapentin would have been considered.

Case two: Cataleyia
Cataleyia, an 11-year-old female, spayed domestic shorthair, presented for intermittent shifting thoracic limb lameness of two months’ duration (Figure 3). She was an indoor-only cat in a single cat household. The owner reported no inciting events that may have caused the lameness. She was otherwise reported to be doing very well at home, with a normal appetite and no evidence of vomiting, diarrhoea, coughing or sneezing. The FMPI clinical metrology instrument was completed and a result of 56% indicated moderate impairment.
Cataleyia had a history of upper respiratory tract infections when she was a kitten, but no other history of significant health issues. She was not taking any medications. Cataleyia was fed a home-cooked diet, which was created by a veterinary nutritionist to ensure that all her nutritional needs were met.
When walking around the exam room, Cataleyia had a relatively stiff gait affecting both thoracic limbs, with limited flexion of the elbows, and a mild left thoracic limb lameness was evident. She readily jumped up on to the examination table, but appeared hesitant to jump off and instead used a nearby chair to assist in her descent to the floor.
A general physical examination was largely unremarkable, except for some minor dental tartar and a moderately increased body condition score (seven out of nine). Cataleyia could be a challenging patient during hospital visits and limited time for handling was anticipated. As it was felt unlikely the entire orthopaedic examination of all four limbs would be completed conscious, the decision was made to perform the thoracic limb examination first so pain responses could be assessed. If time was insufficient to complete the pelvic limb portion of the examination, this could then be performed under sedation later without as much information being lost.
Cataleyia started growling as soon as the orthopaedic examination began, but the growling intensified when her carpi were manipulated in flexion. This then progressed to loud vocalisation when her elbows were extended. Crepitus was palpated in the elbows bilaterally, more severe on the left.
After manipulation of the left elbow, Cataleyia became aggressive, and the remainder of the orthopaedic examination was performed under sedation. Mild thickening and effusion of both elbows was palpable under sedation. Goniometry revealed mildly decreased range of motion of the elbows bilaterally in both flexion and extension. The range of motion of the carpi was within normal limits. No other significant findings were made.
Due to the pain response localised to the carpi and elbows, sedated orthogonal radiographs of all four joints were recommended. Radiographs of the carpi were unremarkable. Radiographs of the elbows (Figure 4) demonstrated smoothly marginated periarticular osteophyte formation bilaterally, more severe on the left.
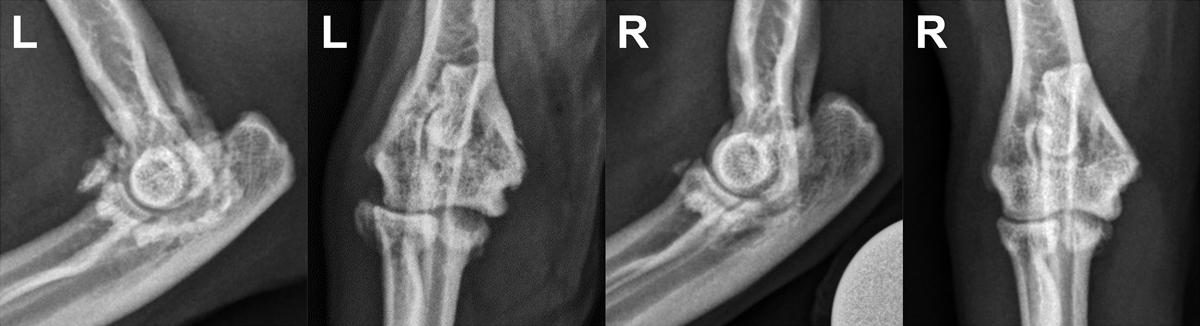
Osteophyte formation was concentrated over the medial coronoid process and the lateral and cranial aspects of the proximal radius. The sesamoid bone within the supinator muscle, visualised cranial to the radial head, was increased in size and remodelled bilaterally, with the changes again being more severe on the left. Peritrochlear sclerosis was evident on the left and subtrochlear sclerosis on the right. Cataleyia was diagnosed with bilateral elbow OA, more severe on the left.
Blood (CBC and serum chemistry) and urine samples were obtained under sedation as well. All results were within normal limits.
After discussion with the owner concerning the radiographic OA present within the elbows, and how this correlated with findings from both the history and orthopaedic examination, an NSAID was recommended to treat the associated inflammation and pain. The owner refused to give Cataleyia any medications unless all attempts at natural treatments failed to control her pain.
An enriching environment is utilised within human medicine as an aspect of chronic pain management (Eccleston et al, 2014; Kroon Van Diest and Powers, 2019); while this has not been explicitly researched in cats with chronic pain, adding cognitive enrichment has been reported to improve health in shelter cats (Gourkow and Phillips, 2016).
Based on the initial conversation with the owners concerning Cataleyia’s disease and their firm objections to drug use, a focused discussion about her environment and normal activity levels took place.
Cataleyia’s owner reported that she tended to sleep the majority of the day in her cat tree, which was near a window. In the early morning and evening, she seemed to enjoy watching, atop her cat perch, the birds in the garden and would chirp at them. She was fed daily at 6am and 6pm in a ceramic bowl near the owner’s dining room table, and they tended to eat together. The owner noted that while Cataleyia had stuffed mice and string toys, she had not been interested in playing with them since she was a kitten. At times, she would meander the house to be near her owner, but she was not noted to have ever been an active cat.
Based on Cataleyia’s current environmental arrangement, her owner had already provided her with many forms of enrichment, including her cat tower, toys, perch, and visual access to nature. However, some additional aspects could be improved upon to give her a more stimulating environment. Hunting is a vital fundamental behaviour often absent for indoor cats (Heath and Wilson, 2014), and so, the use of food puzzles was discussed to accommodate for this.
While Cataleyia’s current home-cooked diet precluded the use of some of the puzzles available, the use of foraging mats and strategy boards was recommended. In addition to providing mental stimulation, these more active ways of procuring food may also help with weight loss (Young, 1997; Clarke et al, 2005).
Weight loss is an invaluable component of any plan to manage chronic pain – particularly in patients with OA. Excess weight places more forces on the joints, which contributes to more pain and lameness, as well as prompting further OA progression (Scarlett and Donoghue, 1998; Simopoulou et al, 2007). Being able to achieve and maintain a lean body condition facilitates control over the pain associated with the condition (Hardie, 1997; Laflamme, 2005; Guck, 2009; Marshall et al, 2010; Rychel, 2010; Monteiro, 2020a).
While the feeding toys may help contribute to weight loss, based on Cataleyia’s higher than optimal body condition score, a more active role was considered likely to be necessary for her to achieve an ideal weight.
To start, a decrease in total caloric intake by 20% was recommended. Suggestions were also made to address Cataleyia’s relatively sedentary lifestyle, including introducing other types of toys, such as a laser pointer, or creating a secure space outside where she may be able to interact more intimately with the environment, such as the birds she enjoys watching. Because it was noted that Cataleyia tended to shadow her owner, it was recommended to use this to our advantage; having the owner be more active within the house could be used to increase Cataleyia’s activity, as well.
Cataleyia’s owner seemed willing to pursue the recommended feeding toys, weight loss and attempts at increased activity, but was also interested in other natural therapies that may help target pain directly. Unfortunately, a paucity of data exists on the efficacy of more holistic treatments for management of chronic pain in cats. Slightly more research is published with respect to the same for human and canine patients.

With regard to nutraceuticals, glucosamine and chondroitin are popular recommendations as part of a multimodal therapeutic plan managing OA; however, even within the human literature, conflicting evidence of efficacy exists (Clegg et al, 2006; Sawitzke et al, 2010; Wandel et al, 2010; Percope de Andrade et al, 2015).
In the veterinary literature, studies investigating the use of such supplements have failed to demonstrate any clinically significant effect (Scott et al, 2017; Cunningham et al, 2022); in fact, a recent meta-analysis showed a very marked non-effect of chondroitin-glucosamine nutraceuticals, leading to a recommendation that such products should cease to be advocated for by veterinary professionals (Barbeau-Grégoire et al, 2022).
Omega-3 fatty acids, however, have been shown to decrease inflammatory mediators and increase levels of anti-inflammatory mediators (Schmitz and Ecker, 2008; Zainal et al, 2009; Zhang et al, 2018). These have been reported to contribute to amelioration of pain in dogs (Barbeau-Grégoire et al, 2022) as well as to improve or eliminate behaviours associated with osteoarthritis-associated pain in cats (Corbee et al, 2012).
Veterinary formulations include gel capsules and liquids allowing accommodation for owner and patient preference; the liquid formulation is designed for garnishing atop of food and should be palatable. The addition of a high-quality, cold processed omega-3 fatty acid was recommended as an adjunct treatment for Cataleyia.
The use of physical rehabilitation, including modalities such as acupuncture, shockwave therapy, laser therapy and massage therapy, is receiving increasing attention in the human and veterinary literature as an adjunctive option for managing chronic pain (Suresh et al, 2008; Anders et al, 2017; Looney et al, 2018; Monteiro, 2020a; Alves et al, 2022); however, no reports on use in cats exist. Due to Cataleyia’s aversion to veterinary hospitals and her disposition within the clinic setting, frequent visits, as would be necessary with the aforementioned therapies, were concerning to the owner, as she did not want to place Cataleyia into stressful situations. She did note that if the current plan did not produce favourable results, she would reconsider her options. A phone conversation was scheduled in one month to reassess Cataleyia’s improvement, or lack thereof, with managing her OA. With remote follow-up, the use of a clinical metrology instrument, such as the FMPI, can be particularly helpful for monitoring improvement.
Follow up for Cataleyia one month later revealed an improvement in her FMPI score, but only to a score of 70%. The owner also reported that the lameness was improved, albeit not resolved, and that Cataleyia had lost a little weight, but not much. At this point, another discussion regarding the potential need to reconsider the use of NSAIDs took place; however, given the initial improvement noted, the owner elected to continue with the current treatment plan for a little longer before contemplating the use of medications.
While Cataleyia’s case is likely not the most common of scenarios, it is important to delve into the more holistic options concerning management of OA-associated pain. In an ideal situation, these would form part of a multimodal therapeutic plan, tailored to the individual patient, being instituted alongside NSAIDs and adjunctive analgesics as necessary.
The cases presented here demonstrate some of the challenges clinicians may encounter when creating an OA management plan for an individual cat, but really only touch the tip of the proverbial iceberg; countless ailments, comorbidities and concurrent medications can complicate this process.
Obtaining a complete patient history, including the use of a validated clinical metrology instrument where possible, and performing a comprehensive orthopaedic examination, will be key for improving diagnosis of this highly prevalent and yet consistently underdiagnosed condition.
Developing a thorough understanding of the treatment options available, including the associated evidence base and the potential for medication interactions, is critical so that owner expectations can be managed, and treatment tailored to produce the best outcome and quality of life for each individual patient.
- Use of some of the drugs mentioned in this article is under the veterinary medicine cascade.
