10 Oct
Cats: surgical management of intracranial meningiomas

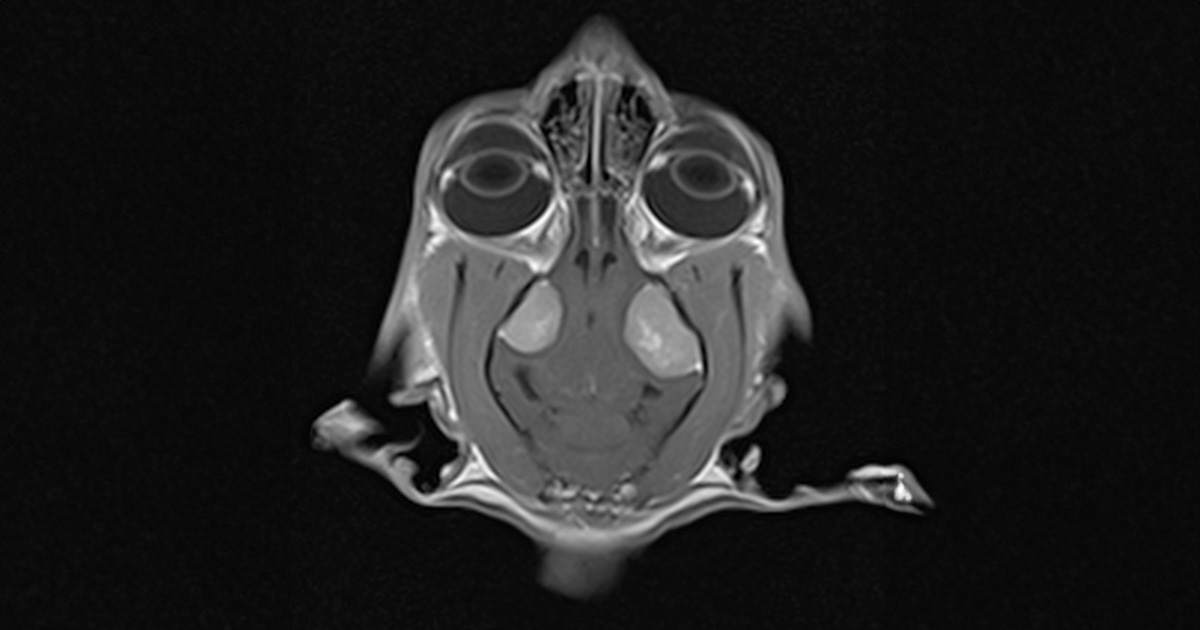
An MRI scan confirmed the presence of two symmetric lesions located in the region of the left and right temporal lobes.
An 11-year-old, female, neutered domestic short-haired cat was referred to Rutland House Referrals for investigation and treatment of seizures, hindleg ataxia, circling and falling over. Orthopaedic and neurological examination was overall unremarkable, apart from very mild generalised ataxia and hind leg weakness. Following a discussion of possible options and complications, the cat was admitted for further investigation.
Patient was admitted for MRI scans under general anaesthetic. An MRI scan of the brain confirmed presence of two symmetric lesions located in the region of the left and right temporal lobes. Both masses were oval shape. Left lesion was noticeable bigger 1.64cm high x 1.8cm long and 1.2cm wide compared to the right sided mass of 1.5cm high x 1.3cm long and 1cm wide.
MRI scan confirmed that lesions were causing severe mass effect resulting in marked transtentorial and cerebellar herniation and an evident dilation of the central canal, extending from the level of C2 to C6. The IV ventricle and part of the third ventricle had features of obstruction on the MRI scan.
Surgical management was elected.
Surgical management
The patient was given on induction a methylprednisolone sodium succinate 10mg/kg IV followed by a 3mg/kg IV phenobarbital and then mannitol 0.5g/kg and lactated Ringer’s solution 3ml/kg/h IV was administered intraoperatively.
Prophylactic antimicrobial therapy was administered on induction and post operatively. Oral cefalexin was administered (20–30mg/kg twice daily, for seven days starting eight hours after the last IV cefuroxime.
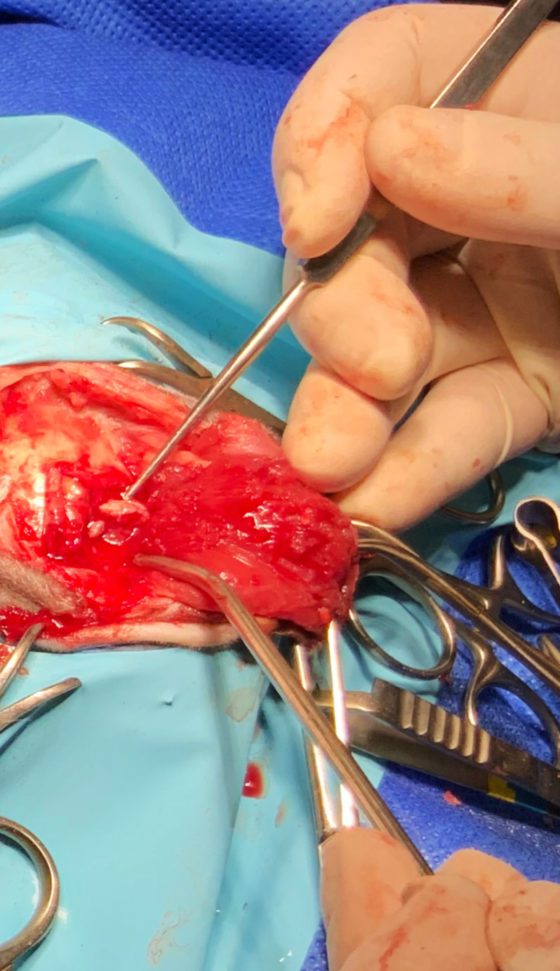
Bilateral rostrotentorial craniotomies were performed. Midline incision was made extending from the level of the lateral aspect of both ears up to the region of external occipital protuberance. Both temporalis muscles were detached from their dorsal and caudal attachments and reflected rostroventrally allowing sufficient temporal bone exposure.
With the use of high-speed spinal drill and Lempert rongeurs, two bone flaps were removed. A bone wax was used to control cancellous bone bleeding and bipolar cautery, to control haemorrhage from meningeal vessels.
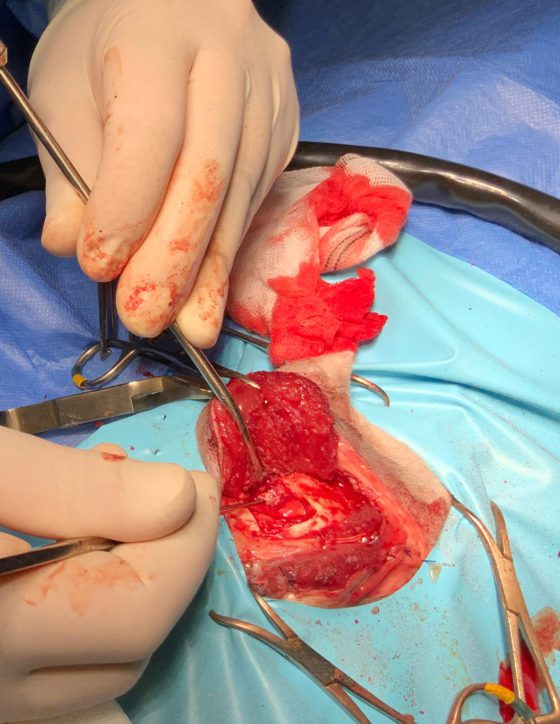
The landmarks and the dimensions of the craniotomies were calculated during the presurgical planning (1.9cm length and 1.6cm width on the left and 1.5cm length and 1.3cm width on the right). Both tumours were removed. Surgicel was used to control bleeding, post-removal of the masses.
Due to the larger size, the left craniotomy was closed using a custom-made titanium mesh; this was fixed to the skull with six self-tapping titanium screws. Both temporalis muscles were reattached back to their dorsal and caudal origin.
Postoperatively, the patient received fentanyl 0.02mg/kg CRI for first 48 hours followed by buprenorphine 0.01mg/kg SC every 6 hours. Lactated Ringer’s solution was continued for another 72 hours. Patient received dexamethasone at the dose of 0.1mg/kg IV SID for seven days.
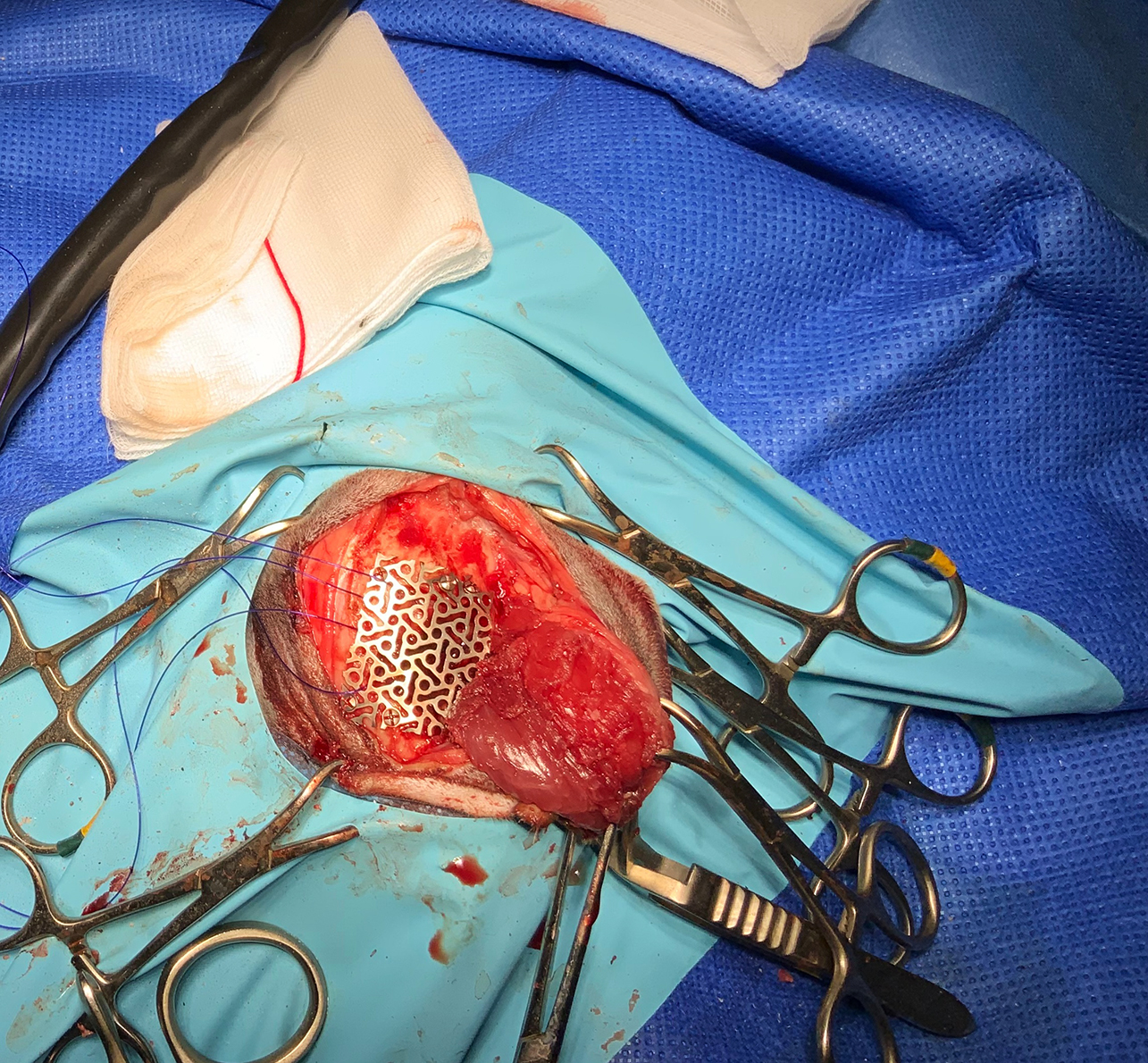
Neurological assessment 24 hours postsurgically revealed moderate bilateral horizontal nystagmus, generalised ataxia, left-sided head tilt, mental dullness and mild head twitching. Seventy-two hours postsurgery, the cat was ambulatory with persistent mild head tilt, relatively normal mentation, minimal horizontal nystagmus and mild generalised ataxia.
Clinical examination on day six revealed no improvement in clinical signs. After nine days of hospitalisation, the owner requested euthanasia due to the inability to provide further supportive care at home. Patient was euthanised despite the advice it was very early in the recovery period. The clinical estimation was that the patient would improve further.
Histopathology
Microscopic examination revealed the presence of neoplastic cells organised in bundles and stream-like structures resting in fibrovascular stroma. Cells contained eosinophilic cytoplasm with round/oval nuclei, stippled chromatin, and had an indistinct margin. Neoplastic cells had an evident feature suggesting presence of anisocytosis and anisocytosis. Cells with pyknotic nuclei were present, and a few areas with no distinct cellular details, being replaced by eosinophilic debris and acicular clear clefts. Microscopic examination revealed a number of lymphocytes, plasma cells, (psammoma bodies) and foamy macrophages with a stroma. Histopathological examination confirmed suspected diagnosis of meningioma (transitional/mixed-type meningioma).
Meningiomas in cats
Intracranial meningiomas are one of the most diagnosed types of primary brain tumours in dogs and cats (Motta et al, 2011). Meningiomas account for approximately 58.1% of intracranial neoplasia in cats (Troxel et al, 2003).
Cap cells present on the surface of the arachnoid villi in the meninges are the primary source of intracranial meningiomas (Kepes 1986; Patnaik 1975). Meningiomas are most commonly found in the mix-breed cat population (Tomek et al, 2006). They are located mainly in the temporal lobes and very rarely in the region of the third ventricle (Saito et al, 2021).
Most of the tumours are solitary, but multiple masses can be seen in 14% to 17% of diagnosed cases (Forterre et al, 2007).
The average age when cats are the most likely to develop meningiomas is 12.5 years (Saito et al, 2021).
The most typical neurological deficits, present in cases of intracranial masses, are: altered consciousness levels; seizures; compulsory circling/pacing; head tilt; and ataxia.
Just over a fifth (21.2%) of cats diagnosed with a brain tumour had no detected specific neurological deficits on the clinical examination (Troxel et al, 2003).
Meningiomas can be treated in several ways, but the most commonly used protocol includes surgical mass removal or debulking surgery. Other treatment options, which can be utilised postsurgery, can include radiothearpy or chemotherapy (Motta et al, 2021; Uriarte et al, 2011).
Surgical management in feline meningiomas has proven to be the most effective treatment option, leading to a much longer survival time compared to other treatment options (Troxel et al, 2003).
For instances of multiple meningiomas, no significant correlation exists between the clinical outcomes and number of meningiomas (Salvati et al, 2004).
However, for cases of surgical management of cases with multiple meningiomas, a higher complication rate can be due to longer aesthetic time, brain parenchyma injuries and intraoperative haemorrhage, (Gordon et al, 1994).
It has been proven that the percentage of mortality and morbidity is dependent on correct craniotomy positioning and topographic knowledge of the brain structures (Mert et al, 2012).
One way to optimise surgical outcomes would be to consider using a patient-specific 3D guide. This technique could be applicable in cases of multiple intracranial meningiomas surgeries – 3D guides can be successfully used to correctly identify the exact surgical site, shorten the duration of the anaesthetic and reduce iatrogenic tissue damage (Singhal at al, 2016).
The recurrence rate of intracranial meningiomas in cats, postsurgical removal, is in the region of 20% (Troxel et al, 2003; Motta et al, 2012).
Patients diagnosed with an intracranial meningiomas may receive palliative treatment, which normally consists of glucocorticosteroids and antiepileptic medications, depending on clinical signs (Fenner, 2000).
Typically, the majority of feline meningiomas are encapsulated and have evident demarcation margins, allowing for relatively easy delineation from the surrounding brain (Gorden, 1994).
In cats, due to their firm and well-defined structure, meningiomas cause compression to the local brain tissue and subsequently cause brain herniation. This is more common in cats than dogs (Troxel et al, 2004).
Feline intracranial meningiomas are much less aggressive in nature compared to meningiomas in dogs (Dickinson, 2014).
Feline meningiomas can be subdivided into five main subtypes: transitional; fibrous; meningothelial; atypical; and anaplastic. Two predominantly seen subtypes diagnosed in cats are transitional (48.9%) and fibrous (33.3%) meningiomas (Saito et al, 2021).
Feline meningiomas display certain histopathological differences compared to canine meningiomas; for example, the presence of cholesterol cleft and focal areas of calcification (Meuten, 2017).
