11 May
Exciting updates in UK radiotherapy for pets

Radiation therapy (RT) – also called radiotherapy – has been used for decades in veterinary medicine.
It can be difficult to access for many. The main barriers in the UK include a combination of logistics (few treatment centres, daily treatments usually required over several weeks), relatively high cost and concern for adverse effects or poor outcome (actual or perceived).
A knowledge gap may exist – in both general practice and specialist centres – regarding which cases may be appropriate candidates for RT (and what type of RT). This is understandable, given most vets have no experience of working in a centre with RT facilities and because radiation oncology is a rapidly developing field.
In addition, the “intensity” of cancer treatment offered or recommended is likely to be very clinician-dependent.
RT is an important component of a multimodal approach to treating cancer in companion animals. Many owners who receive a cancer diagnosis for their pet never get to consult with a veterinary oncologist; therefore, it is important clinicians involved in cancer diagnosis, or treatment have an accurate basic understanding of RT and knowledge of major updates.
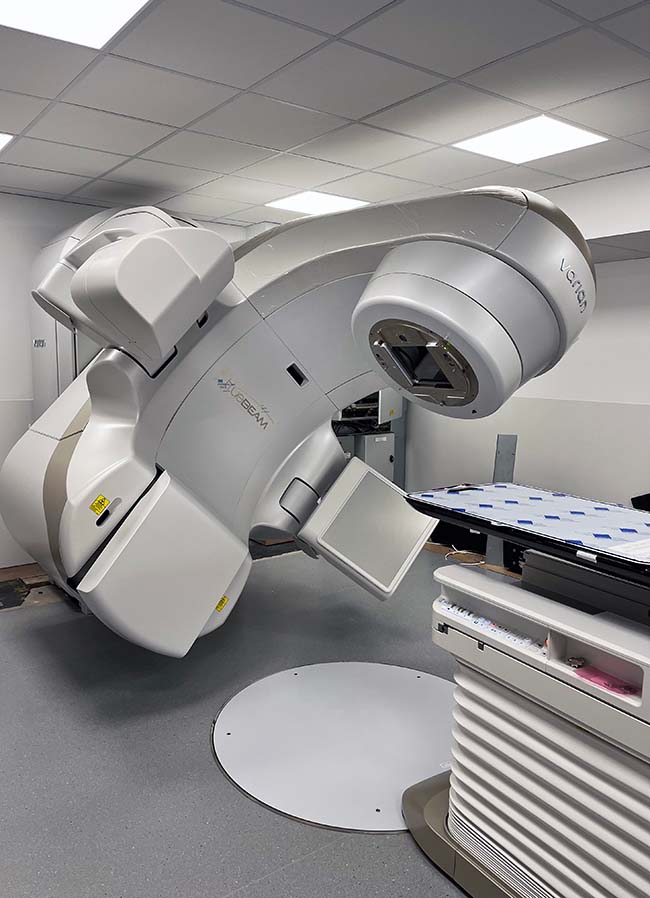
At Southfields Veterinary Specialists in Basildon, Essex, a Varian TrueBeam linear accelerator has just been installed, with various new features that will revolutionise the treatment of pets with radiotherapy in the UK.
Linear accelerator
External beam radiation therapy is the most common type used in veterinary patients, and the therapeutic beams are produced with a linear accelerator (linac; Figure 1).
Linacs produce high‑energy x‑ray (also referred to as photons) and electron beams.
With “megavoltage” photons (most veterinary linacs produce photons with a maximum energy of around six million volts or more), a “skin‑sparing” effect exists because the maximum radiation dose is not deposited at the skin surface. Rather, the dose gradually builds in the first few millimetres of tissue.
As a result of this skin‑sparing effect, less skin reaction occurs compared with older types of radiation, particularly when treating deep lesions (for example, brain tumours) when no/negligible skin reactions should occur.
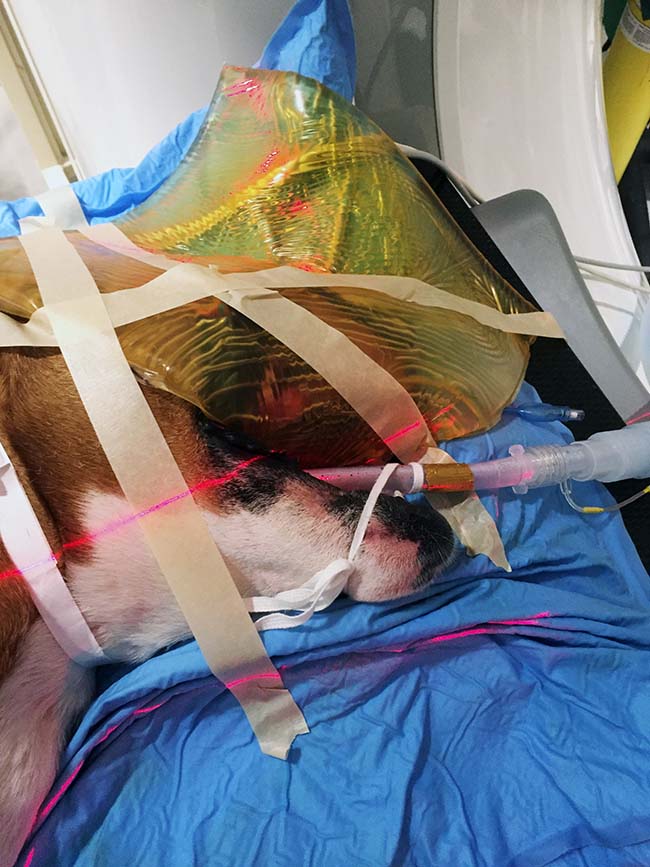
Treatment of superficial tumours or scars requires material with a tissue-equivalent composition (called “bolus”) to be placed over the site to allow the radiation dose to build up so that maximal deposition occurs at the skin surface (Figure 2).
Linacs are isocentric units, meaning the radiation source located in the machine’s head can be rotated 360° around the patient, with multiple beams from different angles converging on a central point within the tumour.
The radiation field size is altered by changing the position of collimators, in the same way as with a diagnostic x-ray machine.
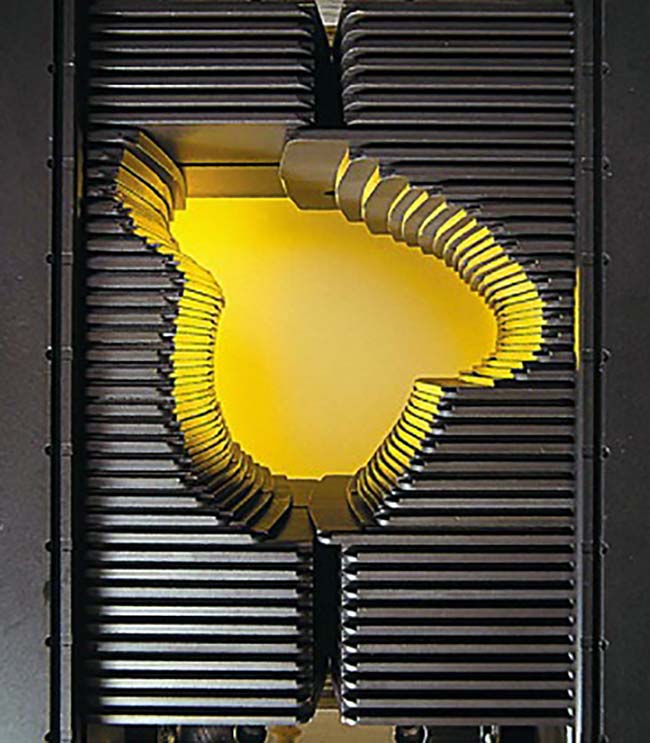
For additional radiation field shaping, modern linacs are equipped with a multileaf collimator (MLC), which consists of a large number of pairs of thick metal rods, with individual motors that drive them in or out of the treatment field, thus creating the desired field shape (Figure 3).
The major advantage of megavoltage x-rays (deep penetration) can also be a limitation with respect to irradiating tumours overlying normal tissues. For example, the treatment of tumours or scars that overlie the thorax or abdomen may result in unacceptable toxicity due to the depth of dose. In these instances, using electrons is often particularly useful.
Linacs with electron capability typically have a range of different electron energies, with differing levels of penetration. Electrons scatter readily in air compared to photons, so beam collimation is extended close to the body surface (approximately 5cm from the patient surface) using electron “cones”. Cones typically provide square treatment fields, so metallic alloy “cut-outs” are used to shape the treatment field (Figure 4).
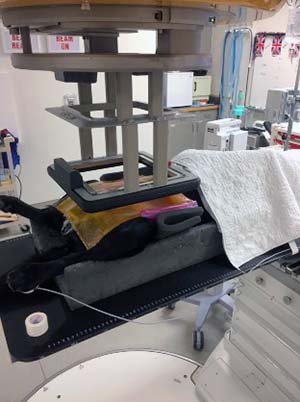
Electron beams are particularly useful for treating lesions that overlie the heart, lungs, kidneys, liver, spinal cord and gastrointestinal tract. The dose falls off rapidly in tissues; therefore, care must be taken to choose the appropriate electron energy to avoid underdosing the deep aspect of the treatment volume.
Additionally, electrons are more prone to be affected by tissue heterogeneities (air, irregular surface contour) and so care must be taken when considering the site to be treated with electrons.
Fractionation
For most patients, the total dose of radiation to be administered is split into multiple small fractions. This is because normal tissue is only tolerant of high total doses if administered as small doses per fraction.
Therefore, this is a means of reducing long‑term damage and late side effects.
As a result, a classical “definitive‑intent” protocol (administering a high total dose to try to get the best long-term outcome) may be 16 to 20 treatments on a once‑daily basis over three to four weeks.
The total dose administered is likely to be in the range of 45 Gray (Gy) to 60Gy, in fractions of around 2.5Gy to 3Gy. For example, common nasal tumour protocols and brain tumour protocols at the author’s institution are 3Gy × 16 (48Gy), and 2.5Gy × 20 (50 Gy), respectively.
Palliative-intent protocols usually involve giving a lower total dose in perhaps three to six fractions on a once-weekly schedule, with a larger dose per fraction (the potential for late side effects is less of a factor as long-term survival is not expected). A common palliative protocol would be 8Gy × 4, weekly (32Gy).
Intensity modulated RT
Intensity modulated RT (IMRT) is a technology that has revolutionised radiation oncology in human and veterinary medicine.
With conventional treatment, each radiation beam in cross‑section is uniform in intensity.
With IMRT, each field can be subdivided into different regions, producing different dose regions and steep dose gradients. This can be achieved by various mechanisms, but mostly involves dynamic movement of the MLC leaves during the delivery of the radiation beam(s), creating a specific dose distribution designed by the radiation oncologist.
Basically, this means the dose can be “painted” on to the treatment volume, while avoiding dose to the adjacent organs at risk.
The massive advantage of IMRT is the ability to sculp the dose to the treatment volume, and avoid dose to adjacent organs at risk (Figure 5). This reduces the risk and severity of acute (short‑term, temporary) side effects, but also the risk of late side effects that can happen months later.
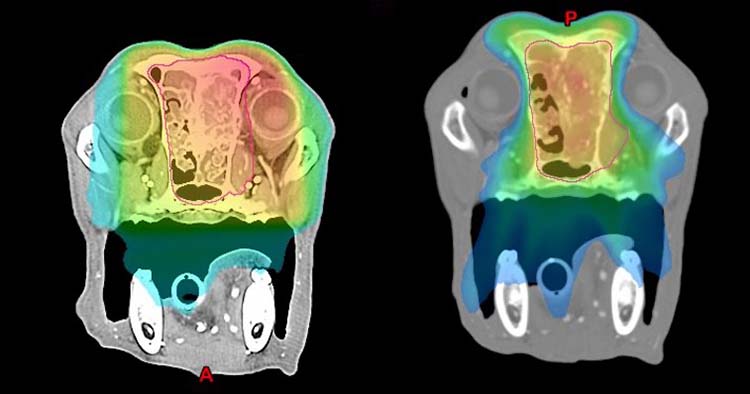
This really comes into its own for nasal tumours, where the eyes, skin, oral mucosa and brain are wrapped around the tumour. This is particularly important in the short term for patients, because acute side effects affecting the eyes (conjunctivitis, keratitis), skin (dermatitis) and oral cavity (mucositis) are significant, albeit temporary.
IMRT can also be used to create differential dose areas; for example, to give a “boost” to the main tumour.
Of course, IMRT is excellent for any tumour that is adjacent to a sensitive structure – for example, cardiac tumours, anal sac carcinoma and bladder tumours.
Image-guided RT
Historically, final patient positioning prior to each treatment was performed by the use of fairly crude methods. Often this relied on small, radio‑opaque markers placed at the time of the planning CT scan, skin marks with a pen and/or bony anatomic landmarks.
While these techniques are still widely used to get the positioning in the ballpark, in most modern veterinary radiation therapy centres, stringent immobilisation and further imaging is performed to confirm accurate positioning prior to dose delivery.
Because it is almost impossible to position a patient perfectly every day, a margin of healthy tissue around the tumour also has to be treated to account for variations in patient position and setup every day. This ensures the entire tumour is treated with confidence, despite some expected malpositioning on a daily basis.
Unfortunately, this means a volume of healthy tissue around the tumour is treated when it doesn’t actually contain cancer. This, of course, contributes to patients’ side effects.
Cone-beam CT scanning (CBCT), incorporated into the linac, is now available. This is an inbuilt imaging unit, producing a CT scan image that can allow for highly accurate patient positioning; not only accurate bony alignment, but also direct tumour visualisation and soft tissue alignment.
The advantage of sophisticated techniques such as CBCT is the confidence that the treatment plan is being accurately delivered. If positioning is known to be highly accurate and confirmed on a daily basis during therapy, the uncertainty margin can be drastically reduced. This means significantly less normal tissue is included in the high-dose radiation field, resulting in fewer adverse effects.
Additionally, it may be possible to escalate the total dose administered, with the hope of improving outcome without increasing toxicity.
Stereotactic RT
Stereotactic RT (SRT) is a relatively new treatment modality in veterinary medicine, the general paradigm of SRT being that:
- Radiation is delivered with extreme precision, and very steep dose gradients (“fall‑off”) are created outside the treatment volume (using IMRT and image-guided RT [IGRT]).
- The number of very high dose fractions are small. For example, a typical nasal tumour protocol would be 10Gy × 3, over three consecutive days. Note the lower total dose than commonly prescribed with definitive‑intent treatment, due to the more damaging effect on the tumour of these very high dose fractions.
- Treatment time is short (usually three to five sessions over one working week).
This is an intense treatment, and is possible only with specific and sophisticated software and hardware, which is expensive.
Confusing nomenclature can arise, including stereotactic radiosurgery (usually defined as a single treatment to a lesion within the CNS), SRT (one to five treatments, anywhere in the body) and stereotactic body RT (one to five treatments to a lesion outside the CNS).
Other than in a few centres (with a specific machine called CyberKnife or TomoTherapy), most SRT is delivered with a linac.
Many basic principles of RT apply when discussing SRT, but some important major differences exist:
- A linac needs to be specially commissioned for SRT, with highly accurate hardware, appropriate software, the ability for image‑guided treatment and submillimetre precision. This involves significant (and expensive) quality assurance and physics support.
- SRT relies on a rapid dose fall‑off outside the treatment volume. The dose drops dramatically from the edge of the treated area, over a matter of millimetres. This helps to spare adjacent tissues from receiving the very high doses per fraction involved in SRT. Therefore, most patients that undergo SRT develop few significant acute side effects.
- Because the dose drops from the prescribed dose to a very low dose over only a few millimetres, it is imperative that the tumour volume is able to be delineated very accurately.
- SRT is usually performed with IMRT technology. This is to minimise significant radiation dose to the adjacent normal tissues.
- Most damaging effects of radiation on tissue can be explained by DNA damage. A different/additional biology may be involved in the success of SRT – namely direct tumour vascular damage and modulation of the tumour-associated immune landscape.
- Because the goal of SRT is to ablate the tumour with a small number of high‑dose fractions and minimise as much as possible the treatment of normal tissues, this treatment is generally only possible for macroscopic tumours. Where surgery has been performed to remove a macroscopic tumour and presumed microscopic tumour cells remain, only fractionated radiation is generally appropriate (because it is mostly normal tissue that is being treated) – although this concept may be challenged more as imaging techniques and SRT evolve.
- SRT can be given with both definitive‑intent and for palliation, depending on the tumour type and clinical context. Many radiation oncologists consider that when used as a palliative therapy, it has a more durable effect than conventional palliative RT, presumably due to its increased dose intensity.
- Case selection for SRT is important, and careful planning and accuracy are vital. As treatment intensity is so high and the dose per fraction is high, potential exists for severe and irreversible late adverse effects, due to substantial normal tissue damage (Figure 6).
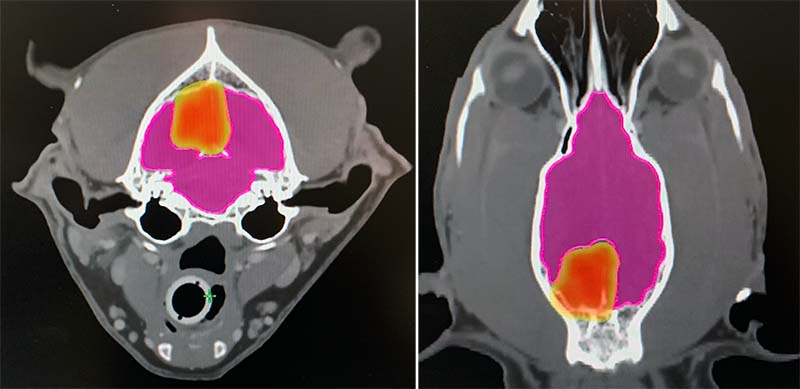
What are the benefits of SRT?
Improved outcome
Some evidence already exists that SRT may result in improved outcomes for dogs with nasal tumours and dogs with certain types of brain tumour. In many other instances, the outcome may not necessarily be better than with conventionally fractionated radiation, but the same outcome may be achieved with fewer treatments and fewer short-term side effects.
Fewer acute side effects
Due to the steep dose gradients at the edges of the tumour, use of IMRT and IGRT, treatment typically has few significant acute side effects.
For example, in nasal tumours treated with SRT, the potential exists for subtle oral mucositis, dermatitis and ocular effects (conjunctivitis, keratitis), but these are typically very rare, mild and totally reversible.
Different biology
The improved outcome in some tumour types could be due to a different mechanism of action.
Improved palliation
Due to treatment intensity, many patients get “better palliation” of signs for those treated with palliative intent.
Less treatment
Less treatment means convenience for owners, and is better for pets.
What are the pitfalls of SRT?
Highly focused
This is a double-edged sword. Some tumours are ill‑defined or may infiltrate microscopically beyond what can be appreciated on imaging studies. Therefore – at least in theory – potential exists for some peripheral tumour cells to be missed.
That said, despite this knowledge and studies in human patients, most recurrences/relapses are still “within-field” as opposed to “marginal miss”.
Increased potential for late side effects
Due to the high dose per fraction (and in some tissues, the intensity of the treatment), the risk of late side effects is generally mathematically much higher than with conventional RT.
However, because with paradigm with SRT is to include as little normal tissue as possible, tissues are protected by avoidance rather than by fractionating the dose, as is the case conventionally.
Unfortunately, it is never possible to include only tumour; therefore, late effects could be more of a problem for certain patients or in particular anatomic sites, particularly if patients survive for a long time or have repeat RT later.
This needs assessing on a case‑by‑case basis.
Brain complications
When brain tumours are treated with SRT, up to approximately 30 per cent of pets may have what are termed “early delayed” side effects, due to some demyelination‑type injury around one to three months following SRT. This usually manifests as recurrence of the original neurological signs.
It is mostly transient and helped with starting (or increased dose of) prednisolone. In a few patients, it can be fatal.
This effect is very rarely seen with conventional RT (fewer than five per cent).
As SRT protocols are refined and experience grows, it is a very realistic goal that these types of effects will diminish over time. For example, when SRT is given over five days, the risk of these effects is very rare in the author’s experience.
Robotic couchtop (six‑degrees‑of‑freedom couch)
Most linac couchtops can move in four dimensions, so positioning errors based on imaging can be corrected and patients can be translated to the appropriate treatment position. However, this does not allow for correction of all possible positioning errors.
A six‑degrees‑of‑freedom (6DOF) couch also allows the additional correction of pitch and roll errors, so patients can be shifted to their treatment position with almost perfect precision (submillimeter). This can all be done rapidly and remotely, without touching the patient.
This makes treatment even quicker, shortening anaesthesia time and contributing to rapid recovery.
Importantly, it also allows the error margin to be reduced even further, meaning even less inclusion of surrounding normal tissue in the treatment field due to the high degree of accuracy.
Respiratory gating
Even with a 6DOF couch, some tumours undergo physiological motion due to breathing (lung tumours near the diaphragm, some body wall tumours, certain vertebral tumours, adrenal tumours and liver tumours).
This makes some tumours “untreatable” with standard radiotherapy options, as so much tissue around the tumour would have to be included – to take this motion into account – that the side effects would be intolerable.
Historically, some institutions have used techniques such as anaesthetic techniques, breath‑hold with neuromuscular blockade or jet‑ventilation techniques – but all can be laborious, time-consuming or make anaesthesia less safe. In some studies, this motion is not accounted for – and it is just accepted that the treatment is not optimal due to respiration.
Respiratory gating means the linac monitors the patient’s breathing with an infrared camera and only treats during specific phases of respiration, when the motion of the tumour is minimal.
Patients can, therefore, be treated quickly, while free-breathing and without the necessity for large error margins.
Southfields is the only centre in the UK with this capability for pets.
Conclusion
The field of veterinary radiation oncology is growing, and many exciting advancements are being made.
As radiation therapy is further explored in the management of cancer, it is hoped the ability to control – and potentially cure – cancer in companion animals will be enhanced.
IMRT can dramatically reduce – or even prevent – acute side effects of radiation, such as oral mucositis or dermatitis. Additionally, where appropriate, SRT can drastically shorten protocols to a few days.
