19 Nov 2018
Pradofloxacin: a feline fluoroquinolone part 1
Lotfi El Bahri, in the first of a two-part article, introduces this antibiotic and its use in cats, including pharmacokinetics, microbiology and mode of action.

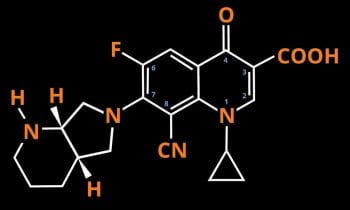
Figure 1. The chemical structure of pradofloxacin. CN = Carbon nitrogen, COOH = carboxylic acid , F = fluorine, H = hydrogen, N = nitrogen, O = oxygen.
Pradofloxacin (PRA) is a third generation veterinary fluoroquinolone with enhanced antibacterial activity, indicated for the treatment of skin and soft tissue infections in cats.
This article will discuss data synthesis on chemical structure, pharmacokinetics, antimicrobial activity and mode of action of PRA, while part two will look at indications, contraindications, drug interactions, resistance to PRA and toxicity in cats.
- Pyrrolidino-piperidine – a bicyclic amine located at position C7. Due to the two chiral carbon atoms of the pyrrolidino-piperidine group, four enantiomeric forms can exist. The S,S-pyrrolidino-piperidine enantiomer is chosen because of its high antimicrobial potency1. The bulky side chain at C7 diminishes the likelihood of bacterial resistance in wild-type bacterial strains and also increases anti-anaerobic activity2.
- A cyano group attached to the C8 – essential for high activity against Staphylococcus aureus and Escherichia coli1.
Physical and chemical properties
Lipid solubility (log P) is a factor determinant that enables drugs to cross cell membranes by passive diffusion. The log P octanol/water values amounted to 1.4 for PRA3.
Absorption
In cats, after oral therapeutic doses of the suspension, PRA is rapidly absorbed from the duodenum and jejunum, resulting in high peak serum concentration of 2.1µg/ml within one hour4,6. The bioavailability of PRA oral suspension is close to 60%6. The bioavailability is reduced in fed cats compared to fasted animals6. Mean peak serum concentrations (Cmaxs) are reduced by 53% by food4,5. PRA plasma concentrations increase linearly with dose4,6.
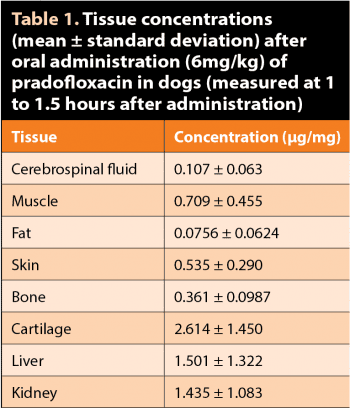
In phagocytic cells, Cmaxs are reported to be six to eight-fold the plasma concentration8. In case of obligate intracellular pathogens (such as Chlamydophila and Mycoplasma), high concentrations of PRA within phagocyte cells are essential. In dogs, PRA is also concentrated in the cartilage (Table 1)9. Small quantities are distributed to the cerebrospinal fluid9.
After oral administration in cats of a single dose of PRA at 5mg/kg, mean maximum concentration (Cmax) of PRA in saliva and tear fluid reached 6.3µg/ml and 13.4µg/ml, respectively – exceeding the concentrations in serum several times within the first hour after administration10. Following the peak, concentrations decreased quickly in tear fluid and, after 10 hours, PRA could not be detected in tear fluid10.
Pharmacokinetic/pharmacodynamic parameters
For fluoroquinolones, the pharmacokinetic/pharmacodynamic parameters that best correlate with clinical efficacy are the ratios Cmax/minimum inhibitory concentration (MIC) greater than 10 and area under the plasma concentration time curve (AUC)0-24/MIC greater than 1259.
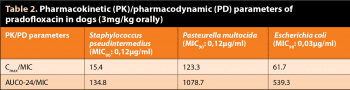
Cmax/MIC and AUC0-24/MIC ratios of PRA calculated for the dose of PRA 3mg/kg orally and based on MIC90 values published by Schink et al (2013) for Staphylococcus pseudintermedius, Pasteurella multocida and E coli are indicated in Table 29,11.
Antimicrobial activity
PRA has a broad activity spectrum including the following.
Gram-positive bacteria
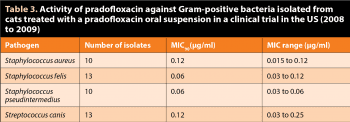
The MIC90 values (MIC at which 90% of the isolates are inhibited) against S aureus, Staphylococcus felis, S pseudintermedius – the most common causative agent in canine pyoderma – and Streptococcus canis isolated from skin infections (wounds and abscesses) in cats in a US field study (2008 to 2009) are listed in Table 35.
Gram-negative bacteria
In a study, 908 bacterial pathogens from defined infections of dogs and cats were tested for their susceptibility to PRA. Pasteurella multocida, isolated frequently from acute skin and soft tissue infections following a cat or dog fighting bite or scratch, Bordetella bronchiseptica and E coli, exhibit low PRA MIC90 values of less than or equal to 0.25μg/ml11. Solely Proteus species and Pseudomonas aeruginosa had higher MIC90 values of greater than or equal to 4μg/ml11.
PRA is also active against intracellular pathogens, such as Mycoplasma species and Chlamydophila species. PRA has anti-Mycoplasma haemofelis effects similar to those of doxycycline – the treatment of choice – and may be more effective at long-term M haemofelis organism clearance than doxycycline12,13. Some doxycycline formulations (particularly the hydrate) have been associated with oesophagitis and oesophageal strictures in cats13. PRA is also active against Chlamydophila felis, causing upper respiratory tract disease and conjunctivitis in cats14,15.
Anaerobic bacteria
PRA shows good activity against various anaerobic bacteria, such as Porphyromonas species, Provetella species, Fusobacterium species, Bacteroides species and Clostridium species. Porphyromonas gingivalis is a major aetiological agent in the initiation and progression of severe forms of periodontal disease.
In a total of 630 isolates, 320 of Prevotella species and 310 of Porphyromonas species isolated from subgingival samples from dogs exhibiting clinical periodontal disease in five European countries, overall MIC90 of PRA was 0.25μg/ml16. No differences in activity were apparent against Porphyromonas and Prevotella isolates16. In addition, PRA showed activity against metronidazole-resistant isolates16.
Rapidly growing mycobacteria
PRA has effective in vitro activity against rapidly growing mycobacteria isolates, such as Mycobacterium smegmatis cluster (n=64) and Mycobacterium fortuitum cluster (n=17) collected from feline and canine patients17.The MIC90 values of PRA are 0.25µg/ml and 0.125µg/ml, respectively17.
- susceptible – less than or equal to 1μg/ml
- resistant – greater than or equal to 2μg/ml
Mode of action
PRA exhibits bactericidal activity. The killing action is of the concentration-dependent type, the rapidity and extent of bacterial killing at the locus of infection are directly proportional to the drug concentration. PRA is a dual-targeting fluoroquinolone18. The first is topoisomerase type II (DNA gyrase) in Gram-negative bacteria and the second is topoisomerase type IV in Gram-positive bacteria19. For old fluoroquinolones, DNA gyrase is the principal target20.
Post-antibiotic effect
PRA can produce significant post-antibiotic effect (PAE), and post-antibiotic sub-MIC (PA-SME) effects on susceptible bacteria, suppressing bacterial growth even after antimicrobial concentrations fall. In vitro, PAE of PRA on S aureus and E coli is two to two-and-a-half hours21.
In vivo (in transudate of dogs implanted SC with tissue cages), PA-SME of PRA on S aureus is 17 hours to 18 hours at half MIC. At three-quarters MIC, killing could be observed up to 10 hours, followed by bacteriostatic activity up to 24 hours21.