3 Jun 2019
Pradofloxacin: a feline fluoroquinolone – part 2
Lotfi El Bahri discusses indications, resistance, interactions, dose and administration, and toxicity of this antibiotic in cats.

Skin and soft tissue infections (wounds and abscesses) in cats are among the most common presenting infections in veterinary consultation, and one of the most common reasons for using systemic antimicrobial therapy.
Early fluoroquinolones approved in the US for use in cats include enrofloxacin, marbofloxacin and orbifloxacin. They have good Gram-negative activity, but are not very active against Gram-positive bacteria1.
![Figure 1. Development of fluoroquinolone resistance (adapted from Zheng [2008]6).](https://www.vettimes.co.uk/app/uploads/2019/06/VT4922_El-Bahri_Fig1-350x168.jpg)
Pradofloxacin (PRA) is an oral third generation veterinary fluoroquinolone, with a broad antimicrobial spectrum – including Gram-positive, Gram-negative, anaerobic bacteria and mycobacteria – indicated for the treatment of skin and soft tissue infections in cats2,3.
Additionally, PRA has low potential for resistance selection in clinically important organisms, as demonstrated by low mutant prevention concentration (MPC) and mutant selection window (MSW) values3,4.
This is the second article providing data for clinical use of PRA in cats – part one (VT48.46) provided data synthesis on PRA.
Indications
PRA is indicated for the treatment of skin and soft tissue infections in cats caused by susceptible strains of Pasteurella multocida, Streptococcus canis, Staphylococcus aureus, Staphylococcus felis and Staphylococcus pseudintermedius3. PRA should only be used as a second line antibacterial, when culture evidence exists that first line drugs will not be effective5.
Resistance
Studies are reporting the likelihood that simply achieving the minimum inhibitory concentration (MIC) of an isolated bacteria may contribute to resistance, whereas achieving the MPC will reduce the resistance.
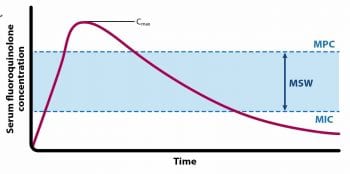
The MPC (Figure 2) is the lowest concentration of drug required to prevent the growth of the least susceptible, first step mutant emerging in a large bacterial population (approximately 1010 colony-forming units)7. For PRA, two resistance mutations would have to occur for bacteria to grow at the MPC (Figure 1)7.
MPC characterises the capacity of an antimicrobial to prevent/severely restrict the emergence of resistant mutants during antimicrobial treatment8. To prevent the selection of resistant mutants, it is necessary to maintain drug concentrations greater than the MPC during treatment.
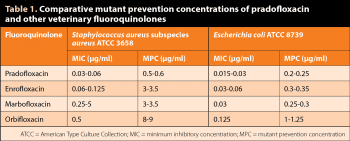
Fluoroquinolones can differ in their ability to select resistant mutants. PRA activity is superior than other veterinary fluoroquinolones, in terms of lower MPC values4 (Table 1). For reference, for S aureus subspecies aureus (American Type Culture Collection [ATCC] 6538), the MPC of PRA is 6 times lower than the MPC for enrofloxacin and marbofloxacin, and 15 times lower than the MPC for orbifloxacin4. Meanwhile, for Escherichia coli (ATCC 8739), the MPC of PRA is 1.2, 1.4 and 5 times lower than the MPC for enrofloxacin, marbofloxacin and orbifloxacin, respectively (Table 1)4.
The MSW (Figure 2) is the antibiotic concentration range between MPC and MIC against the pathogen of interest, and represents the drug concentrations at which resistant mutants are subject to selection6. Fluoroquinolones can differ in ability to select resistant mutants – the narrower the MSW, the lower the likelihood of the potential for resistance selection; the longer the drug concentration remains within the MSW, the greater the likelihood for resistance selection9.
For feline pathogenic E coli isolates susceptible for fluoroquinolones, MSWs are 55µg/ml ± 30µg/ml for PRA and 152µg/ml ± 76µg/ml for enrofloxacin10. Testing against S pseudintermedius showed an MPC90 value of 0.125μg/ml for companion animal for PRA, compared to 0.5μg/ml for enrofloxacin and 1μg/ml for marbofloxacin9.
PRA is also contraindicated in cats with a known hypersensitivity to quinolones. Quinolones should be used with caution in animals with known or suspected CNS disorders.
Drug interactions
Concurrent administration with antacids containing magnesium, aluminium or calcium – or products containing iron or zinc, with sucralfate – cause a marked decrease in the oral absorption of PRA, resulting in lower-than-desired serum levels11.
As with some other fluoroquinolones, concurrent administration of PRA with theophylline may lead to elevated serum concentrations of theophylline and prolongation of its elimination half-life. This may result in increased risk of theophylline-related adverse effects. Fluoroquinolones act through competitive inhibition of cytochrome P450 for the demethylation of theophylline11.
Cimetidine has been shown to interfere with the metabolism of these drugs and should be used with care when used concurrently3.
Concurrent use with oral ciclosporin should be avoided. Concurrent administration of fluoroquinolones may increase the action of oral anticoagulants3.
Dose and administration
The dose for PRA is 7.5mg/kg bodyweight once daily for seven consecutive days3. Each millilitre of PRA oral suspension provides 25mg of PRA3.
To ensure a correct dosage, bodyweight should be determined as accurately as possible. The suspension should be shaken well before use3.
The 15ml size is supplied with a 3ml polypropylene syringe (graduated up to 2ml in 0.1ml steps). Use the syringe provided to ensure accuracy of dosing to the nearest 0.1ml. The syringe should be rinsed between doses.
If acceptable response to treatment is not observed, or if no improvement is seen within three to four days, the diagnosis should be re-evaluated and an appropriate alternative therapy considered3.
Preparations
Polacrilex resin and vanilla flavouring are included in the formulation, to mask the bitter taste of PRA and improve the palatability of the suspension to cats2,12.
Toxicity
PRA has a low acute toxicity – the oral median lethal dose in rats and mice is greater than 2,500mg/kg, and between 500mg/kg and 1,000mg/kg, respectively2,12. In young cats, PRA is well tolerated after a single oral dose of 100mg/kg.
The safety of PRA in cats used for breeding – or that are pregnant and/or lactating – has not been evaluated3,12.
Mutagenicity
In vitro, PRA is positive in Chinese hamster ovary/hypoxanthine phosphoribosyltransferase tests and in chromosome aberration tests, both with and without metabolic activation – indicating a mutagenic potential2,12.
Cytotoxicity/phototoxicity
Tested in vitro in three cell lines – human lymphoblastoma cells from bone marrow, mouse macrophage cells and rat hepatoma cells – PRA was shown to possess a pronounced cytotoxic potential2,12.
Tested in the local lymph node assay of mice and guinea pigs, PRA has a moderate photoreactive potential in guinea pigs2,12.
Adverse effects
The most common side effects in clinical trials of 190 cats included diarrhoea/loose stools, leukocytosis with neutrophilia, a dose-dependent decrease in white blood cell counts, elevated creatine phosphokinase levels, sneezing, haematuria, hypersalivation and pruritus2,12,13.
PRA has no retinal toxic effects in cats2,12. Ocular toxicity was evaluated in 20 healthy adult cats, using PRA in capsules administered orally, once daily, at doses 6 times (30mg/kg) and 10 times (50mg/kg) the recommended dosage during 23 days. No retinal toxic effects were evident – on rod or cone function – under electroretinography evaluation14.
Quinolones have been shown to cause arthropathy in immature animals of various species3. Dogs are particularly sensitive to this side effect3. PRA has shown strong chondrotoxic effects in vitro on canine chondrocytes2,12. At repeat oral doses of 4mg/kg and above, typical quinolone-induced joint lesions were evident2,12.
The use of PRA in cats with persistent joint lesions is contraindicated2,12.
Human warnings
PRA should be kept out of reach of children. Contact with eyes and skin should also be avoided. If contact occurs, eyes should immediately be flushed with copious amounts of water for 10 minutes, or skin washed with soap and water.
Consult a physician if irritation persists following ocular or dermal exposure, or in case of accidental ingestion2,3,12.
Environmental issues
PRA oral suspension is for use for individual animal treatment only. Therefore, its use is not expected to pose a risk to the environment2.
Storage and stability
PRA oral suspension should be preserved at a temperature below 30°C. No special storage conditions are required, but it should be stored in a dry place and protected from light2,3.
When exposed to light in solution, considerable degradation of PRA occurs2.
- Some drugs mentioned are used under the cascade.
- Pradofloxacin: a feline fluoroquinolone part 1.
Latest news

Small animal
Expert Insights: The role of monoclonal antibodies (mAbs) in osteoarthritis (OA) management for pets
Sponsored
10 Mar 2025
