16 Jan 2024
The worms have turned: an update on anthelmintic resistance
Jacqui Matthews explains this issue in horses and offers advice for vets and clients on managing this parasite type.

Image © Patrick Jennings / Adobe Stock
Horses are commonly exposed to intestinal helminths while grazing. Cyathostomins (small redworms and small strongyles) are by far the most common parasitic worms of horses.
Although foals are infected once they start grazing, cyathostomins are most likely to cause disease in one to four-year-olds, in which considerable burdens can develop when they graze contaminated paddocks and are not treated with effective anthelmintics. Adult horses control cyathostomins well (particularly if kept on well-managed paddocks) and are at low risk of developing parasite-associated disease1.
Other common helminths include Parascaris species, which, in foals, can cause weight loss, lethargy, nasal discharge and coughing, and in horses of all ages, the tapeworm Anoplocephala perfoliata, which at higher burdens is associated with colic. In the latter case, burdens of greater than 20 worms have been associated with significant pathology in the intestine2. Unfortunately, anthelmintic resistance threatens to prevent effective control of these parasites.
This article provides an update on anthelmintic resistance, along with an outline of control programmes aimed at protecting the remaining efficacy of the products available.
Anthelmintics and resistance
Four anthelmintic classes are licensed for use in horses in the UK (Table 1). Resistance to all of these is now reported, with the highest prevalence in cyathostomins3,4. A recent review of surveys in 31 countries in the past 20 years identified that, out of 58 studies of benzimidazole treatments against cyathostomins, 100% reported resistance, even in populations described as having limited exposure to this anthelmintic class4.
| Table 1. Anthelmintic classes available for horses in the UK, including efficacy claim summaries as reported in the VMD Product Information Database (www.vmd.defra.gov.uk/ProductInformationDatabase). The current known resistance status for each helminth group is indicated. | |||||
|---|---|---|---|---|---|
| Anthelmintic class – active compound | Strongyloides westeri | Parascaris species | Cyathostomins | Strongylus vulgaris | Anoplocephala perfoliata |
| Benzimidazole – fenbendazole1 | + | + (resistance emerging) | + (resistance common) | + | – |
| Tetrahydro-pyrimidine – pyrantel embonate2 | – | + (resistance emerging) | + (resistance common) | + | + (resistance emerging) |
| Macrocyclic lactone (avermectin) – ivermectin3 | + | + (resistance common) | + (resistance emerging) | + | – |
| Macrocyclic lactone (milbemycin) – moxidectin4 | + | + (resistance common) | + (resistance emerging) | + | – |
| Isoquinoline-pyrazine – praziquantel5 | – | – | – | + (resistance emerging) | |
| Key: +: labelled efficacy, −: no labelled efficacy. | |||||
|
|||||
Cyathostomin resistance to the five-day “larvicidal regime” has also been recorded5,6. With respect to tetrahydropyrimidines, 92% of 31 studies reported lack of efficacy, with resistance to pyrantel salts and benzimidazoles identified together in 19 out of 22 studies. Pyrantel resistance reports vary across regions, being higher in the US (likely associated with availability of a pyrantel pamoate preparation marketed for daily in-feed use). In the UK, lower levels of cyathostomin resistance to pyrantel embonate are reported7,8.
Perhaps most concerning are emerging reports of cyathostomin resistance to macrocyclic lactones. For many years, ivermectin/moxidectin resistance – as measured by faecal egg count (FEC) reduction tests – was rarely reported, with isolated descriptions from Brazil9. However, FEC reductions below the accepted threshold for efficacy have now been observed in yearling groups in the UK8,10 and overseas11.
These reports add to the many that describe shortened cyathostomin egg reappearance periods (ERPs) after treatment with moxidectin, which was originally observed to suppress egg shedding for 22 to 24 weeks12.
Many studies now demonstrate short ERPs (approximately four weeks) for both macrocyclic lactone compounds, and the likelihood of finding this feature in cyathostomin populations in the UK is high13. With increasing incidences of reduced efficacy of all compounds against cyathostomins, multi-class resistance is a real threat to the effective control of these parasites.
Resistance is commonly found in Parascaris species – especially to ivermectin; of 29 publications that evaluated macrocyclic lactone treatments, evidence of resistance was shown in all studies4. Resistance to the other anthelmintic classes is reported at a lower rate in Parascaris species. For example, pyrantel and benzimidazole resistance was observed at 25% and 23% prevalence rates in 16 and 13 studies, respectively4.
Multi-drug resistance is less commonly reported in Parascaris species compared to cyathostomins, but this could be due to the lower number of surveys that have investigated this.
In 2023, treatment failure of both anthelmintics licensed against A perfoliata was reported in the US14. Here, tapeworm eggs were observed in faeces after treatment of yearlings with pyrantel or praziquantel, and after praziquantel treatment of mares on the same premises. Although FEC tests are generally insensitive for detecting A perfoliata due to intermittent proglottid shedding and because many infections comprise a large proportion of immature or sterile worms15, the finding of tapeworm eggs in faeces a few weeks after treatment indicates emerging signs of yet another equine parasite, against which anthelmintic treatments may be suboptimal.
For all helminth species, the preservation of remaining anthelmintic efficacy is now paramount in control programmes.
Anthelmintic resistance selection
Helminth populations are extremely large and genetically diverse, so they possess a strong capacity to adapt under selection pressure3. Anthelmintic treatments that kill helminths at a rate of 90% to 99% function as a potent trigger for selection of resistant genotypes (Figure 1).
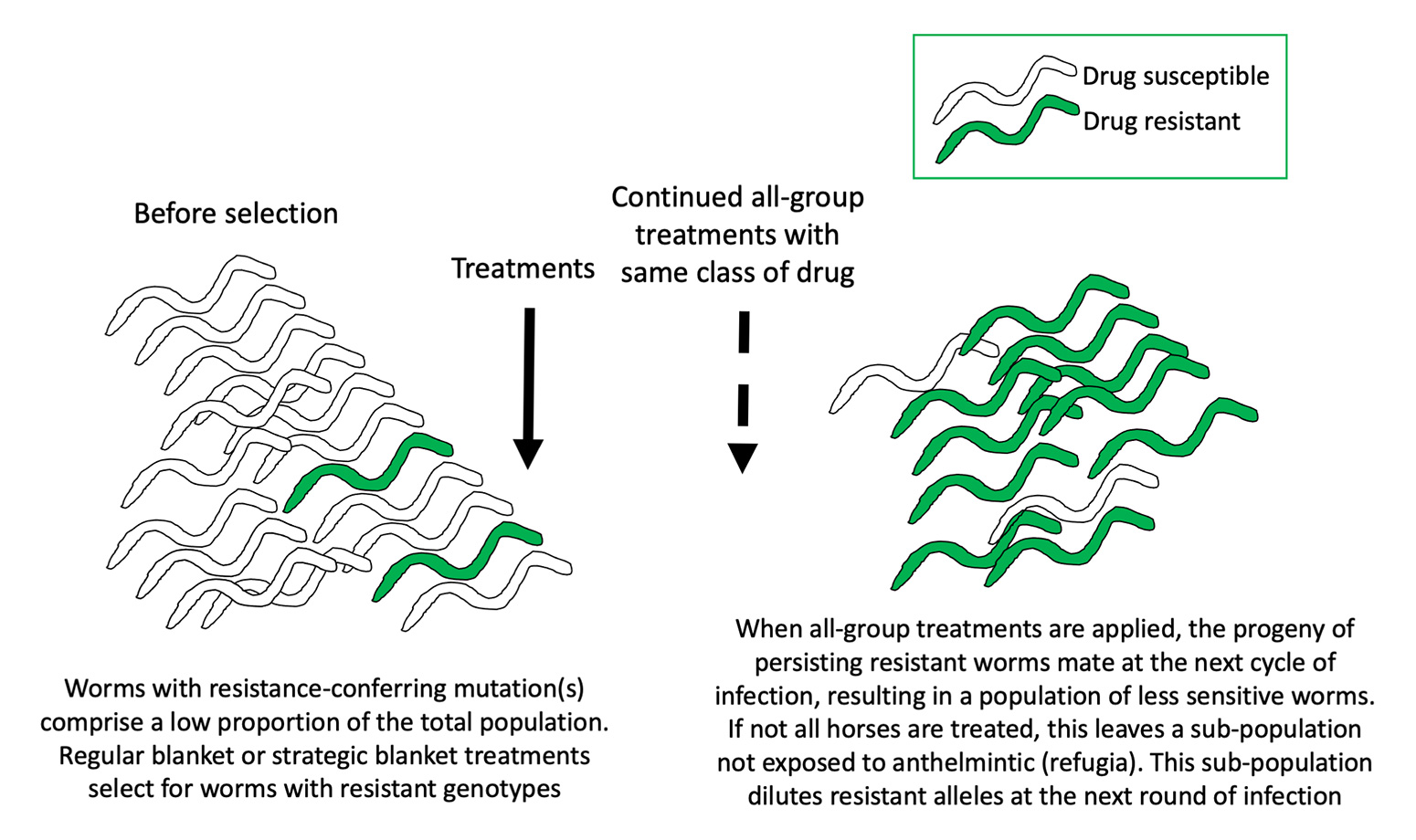
Although mechanisms of resistance in equine helminths are not well-characterised, experimental infections with sheep nematodes have shown that resistance can evolve within a few generations when reinfection occurs with worms exposed to gradually increasing concentrations of drug16. Although the development rate is likely to be slower in the field, the sheep experiments demonstrate key principles as to how parasitic worms acquire resistance. Moreover, many common practices accelerate the rate of resistant genotype selection:
- Regular worming of all animals with no attention paid to refugia (those worms not exposed to drugs at each treatment).
- Suboptimal dosing due to inappropriate weight estimation.
- Treating all animals and moving to clean pasture so that the only worms contaminating new paddocks are those that have just been through a round of anthelmintic selection.
- Using inappropriate product/administration routes for a product that is not licensed.
Resistance is difficult to address as it is not obvious phenotypically (as detected by lower FEC reduction/clinical disease in treated animals) until a relatively high proportion of the worm population contains drug-resistant alleles.
Little is known of the genetic mechanisms in equine helminths (with the exception of benzimidazole resistance), so molecular tests cannot be designed to detect it at an early stage. This is compounded by reversion to susceptibility being unlikely17 and the possibility of side resistance between compounds with a similar mode of action (ivermectin/moxidectin), although this is yet to be proven in equine nematodes.
With no new anthelmintics with unique modes of action coming to market soon, it is essential that herd drug resistance profiles be assessed so that owners, instead of applying treatments blindly, are aware of which anthelmintics they need to stop using and which ones to preserve. Resistance development is insidious, which means that anthelmintics may apparently continue to provide clinical efficacy when FEC reductions two weeks post-treatment are less than 85/90% (pyrantel embonate/fenbendazole) or less than 95% (ivermectin and moxidectin).
In sheep, it is proposed that when nematode FEC reduction is less than 80%, a clinical effect is observed18. For these reasons, it is likely that resistance is not detected clinically until it is at a high level in the incumbent worm population.
Detecting low efficacy as early by employing regular FEC reduction tests will help in the design of control plans that decelerate further selection. To do this, perform FEC analysis just before treatment and 14 days later. The mean percentage FEC reduction for the group at day 14 will provide a measure of effectiveness of the product administered.
Generally, if the mean FEC reduction is less than 85% after pyrantel embonate treatment, less than 90% after fenbendazole treatment or less than 95% after macrocyclic lactone treatment, resistance should be considered.
Addressing resistance
With no prospect for new anthelmintic classes in the foreseeable future, helminth control must not rely on these alone. Sustainable control can be achieved by improving pasture management and, depending on risk, applying strategic treatments or diagnostic-led treatments (Figure 2).
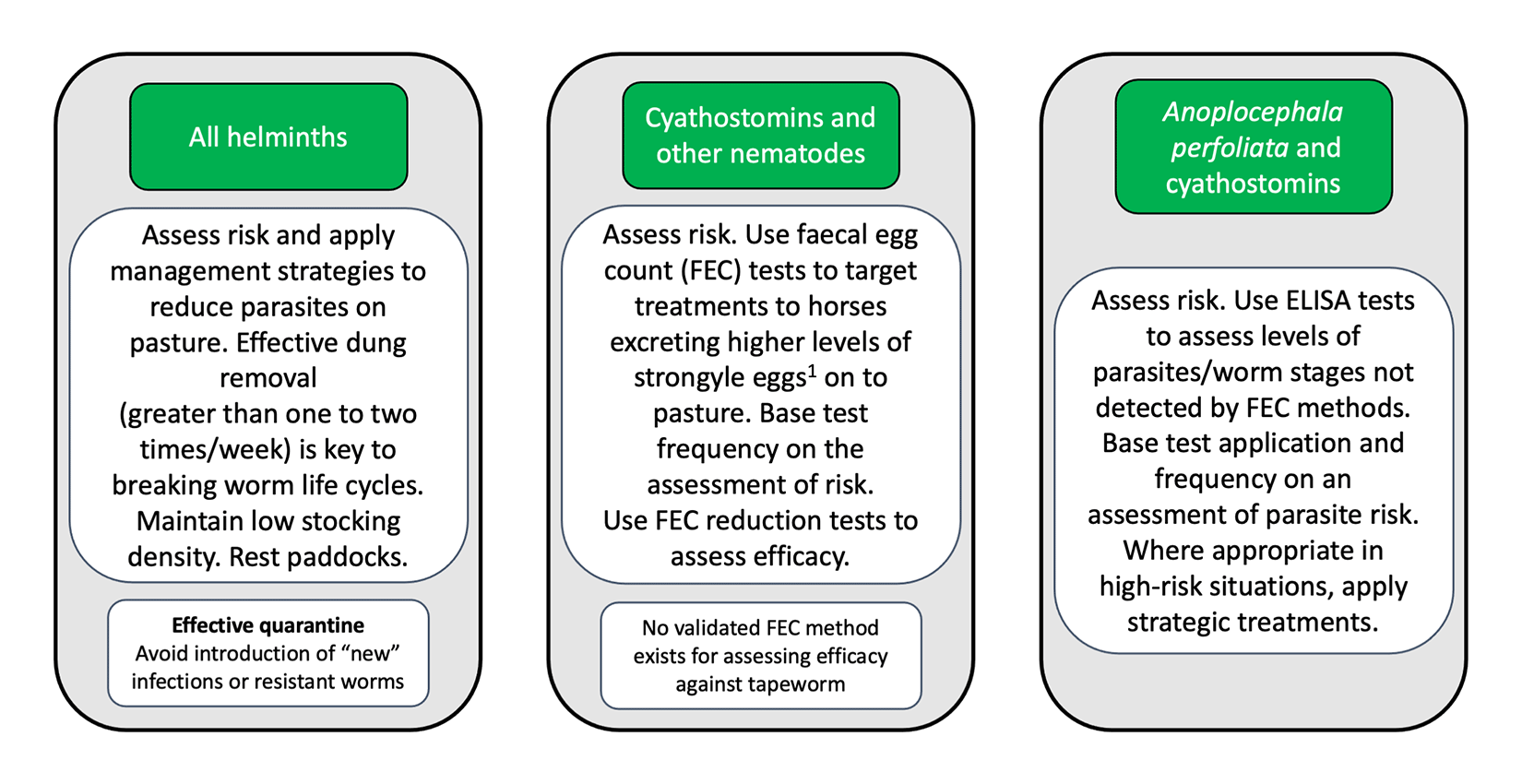
Although all horses have potential to carry worms and contaminate pasture within a group on well-managed paddocks, most tend to have low-to-moderate burdens and only a few will have many worms. This means that diagnostics employed to target only horses with higher burdens (for example, those in treatment recommendation categories in ELISA tests) or egg shedding levels (for example, those shedding greater than 200 strongyle eggs per gram) for treatment can considerably reduce anthelmintic use, therefore preserving refugia. Younger horses with higher worm burdens/egg shedding may need more frequent testing and, in some cases, strategic treatments (for example, encysted cyathostomin treatment in autumn/winter).
Programmes must incorporate excellent pasture management to reduce infection load – especially on paddocks grazed by younger groups. The most effective way to reduce contamination is to remove dung from paddocks at least one to two times a week. This is relevant for both nematodes (to reduce translation of third-stage larvae on to grass) and for cestodes (to reduce oribatid mite contact with tapeworm eggs).
Dung should not be placed near paddocks because oribatid mites and nematode larvae can migrate back on to grazing. Spreading fresh manure on to grazing paddocks is inadvisable and harrowing only serves to spread parasites around in weather conditions experienced in the UK.
Other management methods that help reduce pasture contamination include maintaining a low stocking rate (a maximum of one to two horses per acre) and resting paddocks. For cyathostomin control, keep horses off paddocks from the end of one season/year until six months into following season/year. Grazing with non-equine species can be combined with resting paddocks because ruminants function as “biological vacuums” that reduce infection load of equine-specific helminths.
For all incoming horses, it is important to assess infection risk and apply a “test and treat” or (appropriate) treatment approach. It is recommended to assess effectiveness of anti-nematode treatments by a FEC reduction test. A management-based/diagnostics-led control programme for adult horses is summarised in Figure 3.
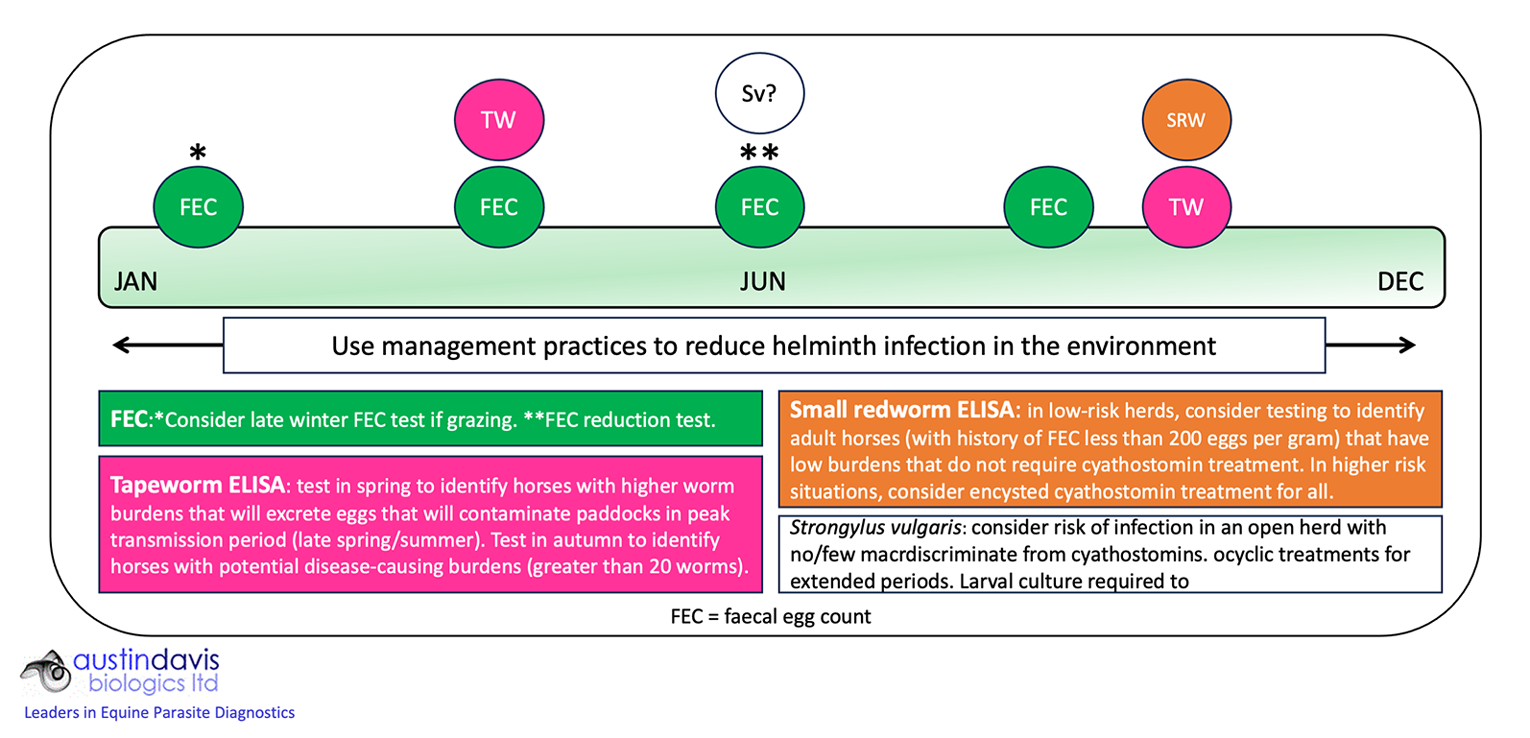
Testing identifies which horses require treatment, with FEC methods applied to assess efficacy of anti-nematode anthelmintics (fenbendazole, pyrantel, ivermectin and moxidectin) in summer when FECs are likely to be higher. If greater than 30% of horses test positive in coprological-based or antibody-based tests, paddock management requires improvement to reduce infection risk.
If tests indicate no indication for nematode treatment, praziquantel (available as an extemporaneous formulation for veterinarians to prescribe under the cascade system) should be administered to avoid unnecessary selection for resistance in the other species.
Latest news

Related clinical resources
Diagnosing arthritis in cats
Clinical Assist
