10 Mar
Tumour margins – quantity vs quality

Figure 2. The narrowest skin margin should be taken as your overall margin – for example, no point exists in taking 2cm in one direction if only 5mm can be achieved in another direction.
As a wise oncologist recently said, a histological margin is either complete or incomplete and no other definition will do.
This sounds easy, but how do we make a decision about what margins to take, and what options do we have to achieve this?
This article aims to review decision-making when planning surgical margins to maximise patient outcomes.
Adjuvant: non-surgical treatment options performed following surgery – for example, chemotherapy or radiotherapy.
Neoadjuvant: non-surgical treatment performed prior to surgery with a view to improving the chances of achieving a curative intent surgery (usually to reduce the size of the lesion).
Incisional biopsy: a sample taken from a lesion to achieve a histological diagnosis prior to surgery.
Excisional biopsy/marginal excision: removal of the lesion with a minimal margin.
Curative intent surgery: one where margins are planned to maximise the chances of removing all neoplastic cells in the local area.
Cytoreductive surgery: one where adjuvant treatment – such as radiotherapy – is planned at the start of any treatment, and surgery is therefore performed to remove as many neoplastic cells as possible while still achieving a primary closure of the incision and minimising wound healing complications.
Primary closure: one where the skin edges can be apposed postoperatively without the need for skin flaps, free skin grafting or second intention healing.
Step one: what is your diagnosis?
Sampling a lesion prior to surgical planning should be considered essential. This can be a hard sell for some clients, but the recommended staging protocol and surgical margins often vary significantly from tumour type to tumour type, and scrimping on pre‑surgical biopsy can be a false economy.
For the majority of tumours, a diagnosis can be achieved by fine needle aspiration, but what happens when this is unsuccessful – for example, with soft tissue sarcomas?
Under such circumstances, the surgeon should consider whether a preoperative diagnosis will affect treatment planning. If the answer is yes, biopsy of the lesion should be considered.
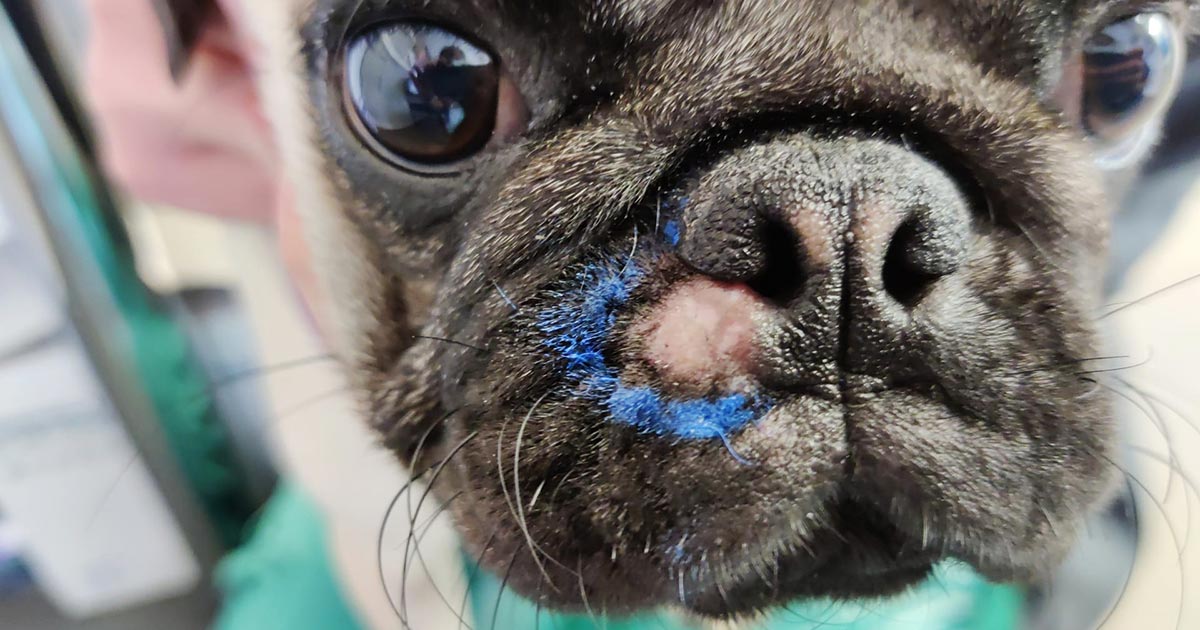
In such cases, an incisional biopsy or Tru‑cut biopsy represents the best option for achieving a diagnosis where cytology has failed, as long as the site of the biopsy will not affect your overall planning for the definitive surgery (Figure 1). Practically, this means taking your biopsy from the centre of the lesion – either using a biopsy punch or as a wedge biopsy – therefore ensuring the biopsy tract can be removed without affecting your margins for the definitive surgery.
Ensure you take a deep enough sample to ensure you have biopsied the lesion rather than the skin and SC fat.
Excisional biopsy (removal of the gross extent of the lesion with no planned margin) is usually reserved for lesions where knowing the tumour type prior to surgery would not affect decision‑making with respect to staging or surgical planning.
Several specific circumstances exist where it may be considered too risky to biopsy a lesion or where biopsy of a lesion will not affect the surgical decision‑making for that patient – for example, a bleeding splenic mass leading to a haemoabdomen (as “excisional biopsy” of the spleen will be the only option).
In some clinical cases, a need for a clinical judgement will inevitably be based on risk versus benefit of the sampling technique. One such example is ultrasound‑guided aspiration of adrenal lesions in dogs, for which a 1% risk of mortality has been approximated (Pey et al, 2020); would knowing the lesion type affect your surgical planning?
Step two: staging
Knowing a diagnosis prior to surgery will also help you to predict the behaviour of the lesion and therefore dictate what staging is required.
Staging no longer consists solely of obtaining thoracic radiographs – and with increasing access to advanced imaging and ultrasound in first opinion practice, we are moving towards a more thorough approach to staging prior to surgery.
So, why bother? Ultimately, in many types of neoplasia, the presence of metastasis will affect prognosis, indications for adjuvant therapy and the appropriateness (or not) of performing a more invasive curative intent surgery.
As an example, recent literature supports the hypothesis that a significant rate of occult locoregional metastasis exists in canine mast cell tumours and removal of metastatic lymph nodes at the time of surgery can significantly improve prognosis (Marconato et al, 2018; Sabattini et al, 2021). Therefore, assessing and – if possible – aspirating or extirpating locoregional lymph nodes is of benefit.
Liver and splenic aspirates are also recommended in canine mast cell tumours as ultrasound alone appears to carry a low sensitivity and specificity (Pecceu et al, 2020).
Step three: what margin do you need?
Why do we need to take a surgical margin? In some cases of benign lesions – for example, a lipoma – a marginal excision will suffice and no planning is required.
For locally infiltrative lesions, a margin of “grossly normal” tissue is usually recommended to increase the likelihood of removing all the neoplastic cells from the local area.
In high-grade soft tissue sarcomas, the likelihood of recurrence or distant metastasis is high regardless of the margin you achieve at surgery; adjuvant therapy would therefore likely be recommended regardless.
It is important to remember that our planned margins may not be those achieved at surgery. Surgical margin length reductions occur due to a combination of physical factors (such as tissue elasticity, myofibril contraction and histological processing) and biological factors (such as microscopic tumour infiltration into the grossly normal surgical margin; Milovancev et al, 2018).
Guidelines often exist for what margins should be attempted for a specific neoplasm. For example, a minimum of 3cm to 5cm of grossly normal intestine orad and aborad to the lesion is recommended when removing a neoplastic lesion from the small intestine, whereas in canine dermal mast cell tumours we often attempt to achieve a 2cm lateral margin with a deep fascial plane, although other systems have been proposed (Pratschke et al, 2013).
Quality versus quantity
Not all tissues are equal when deciding on surgical margins. For example, a thin layer of fascia is a much better barrier to the passage of neoplastic cells than fat cells, which are more loosely apposed.
Understanding the biological behaviour of your lesion prior to surgery is also helpful in determining margins.
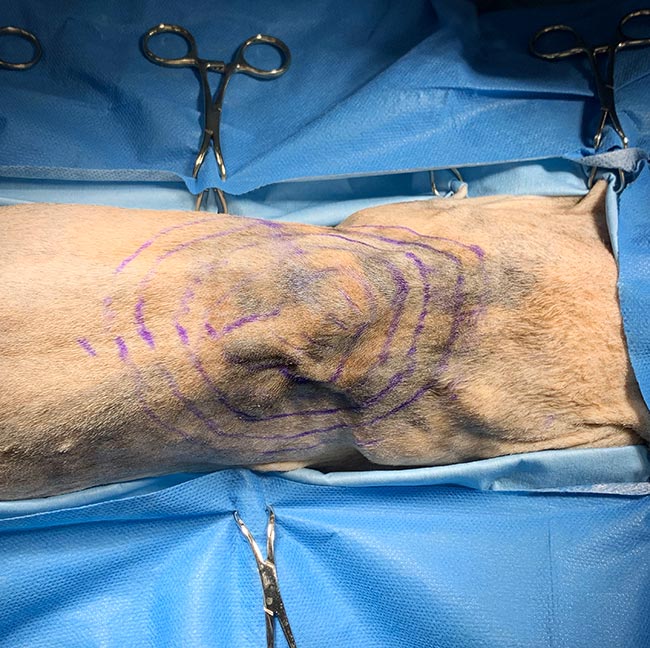
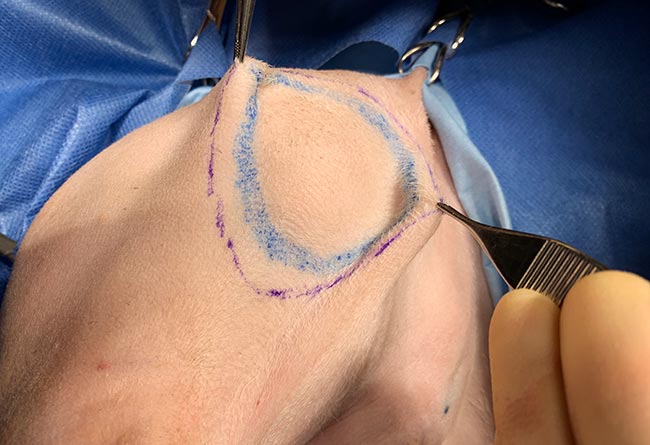
Your narrowest margin should dictate the overall margin. What is meant by this? Ultimately, no point exists in taking a 3cm margin in one direction if you can only achieve a 5mm margin in another. Facial reconstruction is a good example of this – the margin abutting the ventral part of the nostril will dictate the overall lateral margin taken (Figure 2).
Intraoperative use of a skin pen allows you to mark out several margins prior to starting surgery (Figure 3). Forceps can be used to pick up tissue at the marked edges to assess skin elasticity and the likelihood of achieving a primary closure (Figure 4).
Step four: surgical planning with respect to tumour margins
Once we know what margin we would like to achieve, our next question is whether it can be achieved without an unacceptable risk of morbidity and mortality to the patient, considering the results of staging.
Ultimately, does the benefit of surgery outweigh the risks?
For instance, an invasive surgery (carrying a high risk of faecal incontinence due to the size of the primary mass) for management of stage four anal sac adenocarcinoma (where extensive metastasis is present) may be considered inappropriate, whereas resection of a feline injection site sarcoma with a 4cm margin by an experienced surgeon is likely to be achieved without significant patient risk (assuming no other comorbidities; Figures 5 and 6).
What questions should we be asking?
- What are the recommended margins for this tumour type?
- Can such a margin be achieved?
- What would be the likely morbidity and mortality of achieving a curative intent surgery for this neoplasm in this patient? Can a primary closure be achieved, or will a more complex solution be required for reconstruction – and if yes, will this affect the timing of any adjuvant treatment?
- Does the patient have other comorbidities – for example, severe OA affecting all four limbs precluding limb amputation in canine appendicular osteosarcoma?
- Is there a role for adjuvant therapy (such as radiotherapy) to reduce patient morbidity?
- What is the owner’s financial situation? This may affect willingness to pursue a more invasive surgery or adjuvant therapy such as radiotherapy.
Step five: the role of neoadjuvant therapy
In cases where a primary closure cannot immediately be achieved, neoadjuvant therapy can be considered to try to reduce the size of the lesion prior to surgery.
In its simplest form, this can be the administration of prednisolone and antihistamines in canine mast cell tumours, but can also include more traditional chemotherapy agents such as vinblastine.
Response to such therapies will vary depending on the tumour type and the grade of the tumour; therefore, use of such treatment strategies should be discussed with an oncologist where possible.
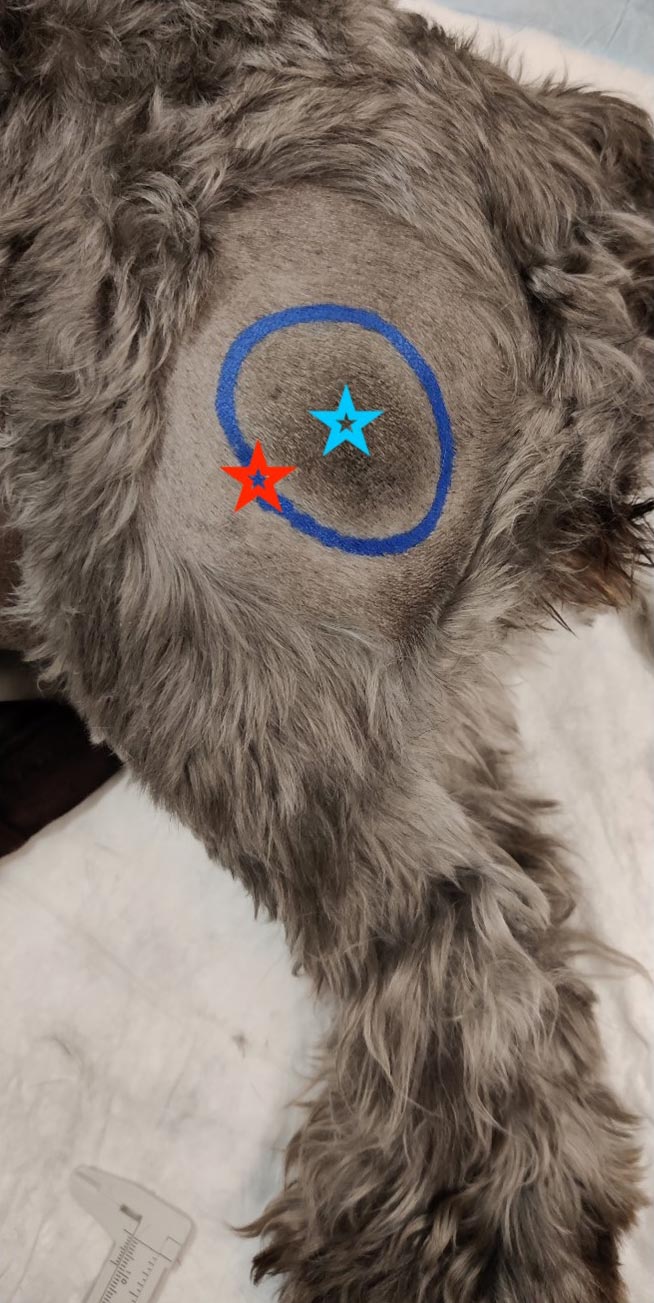
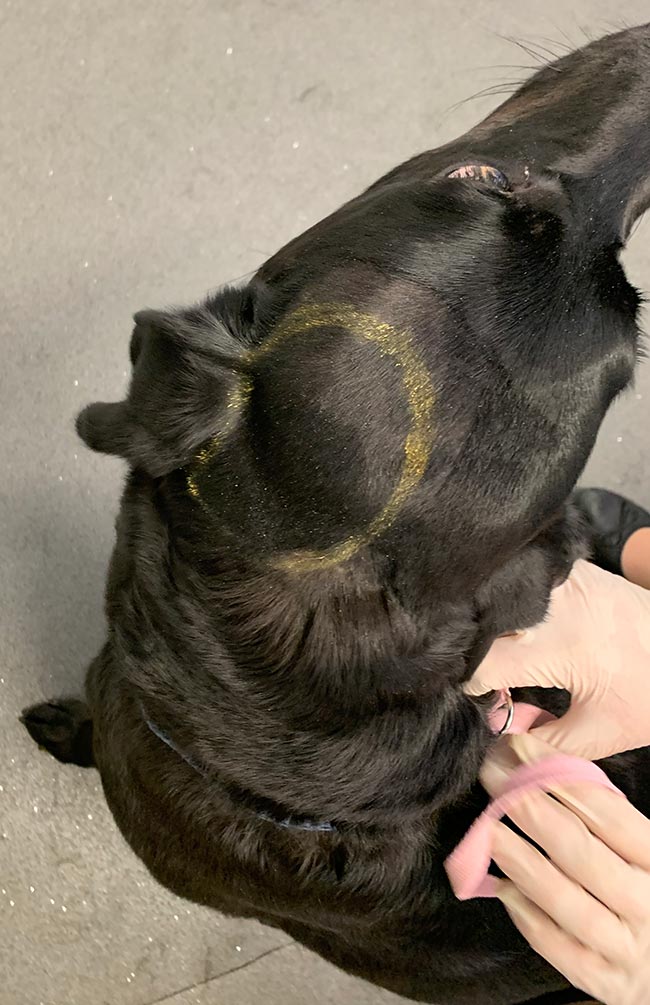
The dimensions of the lesions should be measured prior to and following adjuvant therapy to objectively monitor response. This can easily be achieved in dermal or SC lesions as the gross extent of the lesion can be measured with callipers and circled with a permanent marker after clipping the fur (Figure 7), but may require advanced imaging for monitoring of intracavitary lesions such as a thymoma.
Step six: making friends with your pathologist
Pathologists are smart people, but they are not mind readers – and the more information we can provide, the better the information they will give us in return.
So, what information are we talking about?
- location of the lesion
- rapidity of growth
- any cytological diagnosis
- the results of any staging performed
- what you were aiming to achieve when you took this sample – for example, incisional biopsy versus curative intent surgery
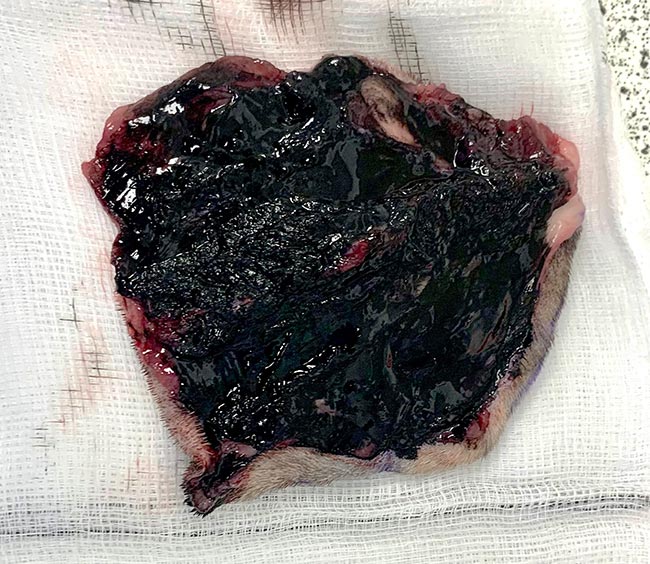
Inking of the margins of your sample (Figure 8) is useful as it will indicate to the pathologist the clinical margins as determined by the surgeon prior to fixation in formalin, due to the discrepancy often seen between the clinical margins and the histological margins (Risselada et al, 2015).
In return, based on this information, the histology report should provide a diagnosis (if possible; even histology is not infallible), margin assessment (complete versus incomplete with numerical values) and information on the behaviour of the tumour (grade/mitotic index and so on).
Summary
- Always know what you are dealing with before removing it.
- Staging is important as it will affect treatment outcomes.
- Make sure the owner knows the whole treatment plan before you start. For example, if you are planning adjuvant therapy make sure the owner is aware of the cost and pros/cons of this before removing the mass.
- Give your pathologist a fighting chance by giving adequate information and contact him or her to discuss cases if the result is not as expected.
• Some drugs mentioned in this article are used under the cascade.
