11 Nov 2022
Urine sediment findings in a dog

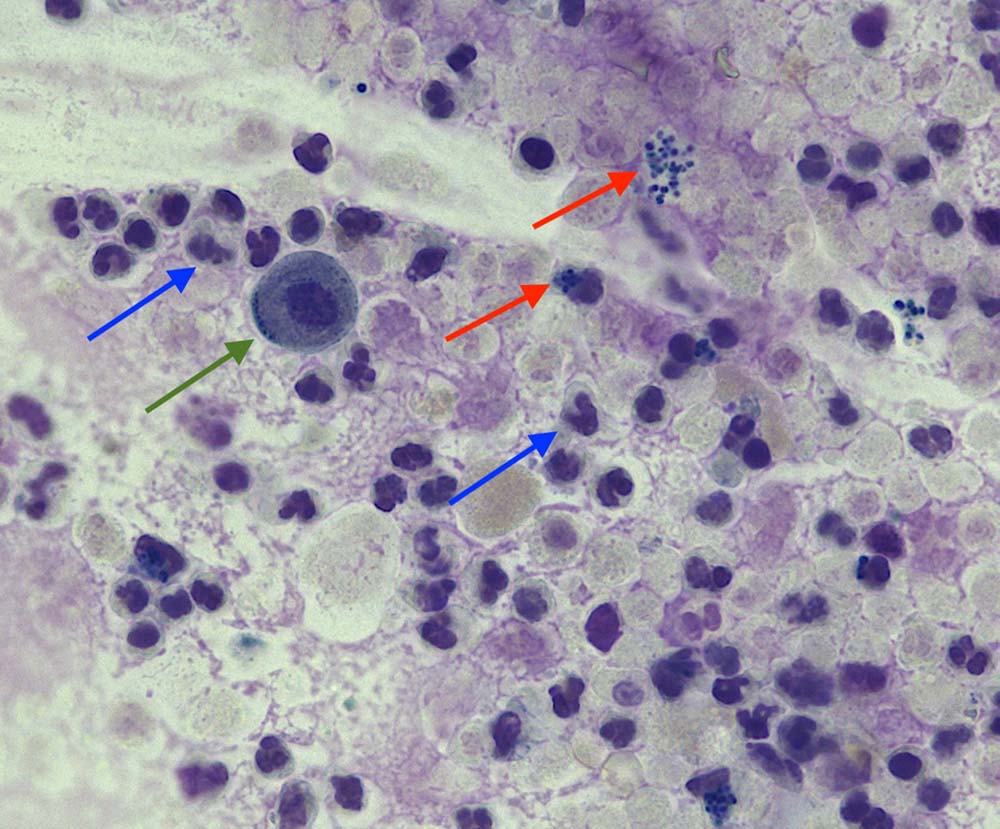
Figure 2. Stained urine sediment from a dog (Wright-Giemsa, 40×).
A six-year-old female Labrador retriever was presented to the veterinarian for a week’s history of pollakiuria (signs of urgency and frequent urination) and stranguria (straining to urinate).
A fresh urine sample was collected by cystocentesis into a plain tube and submitted to an external laboratory for analysis. Urine sample was concentrated (urine specific gravity; USG 1.039), and dipstick was moderately positive (++) for blood and protein.
Concentrated urine sediment preparations were made, both unstained (Figure 1) and stained (Figure 2).
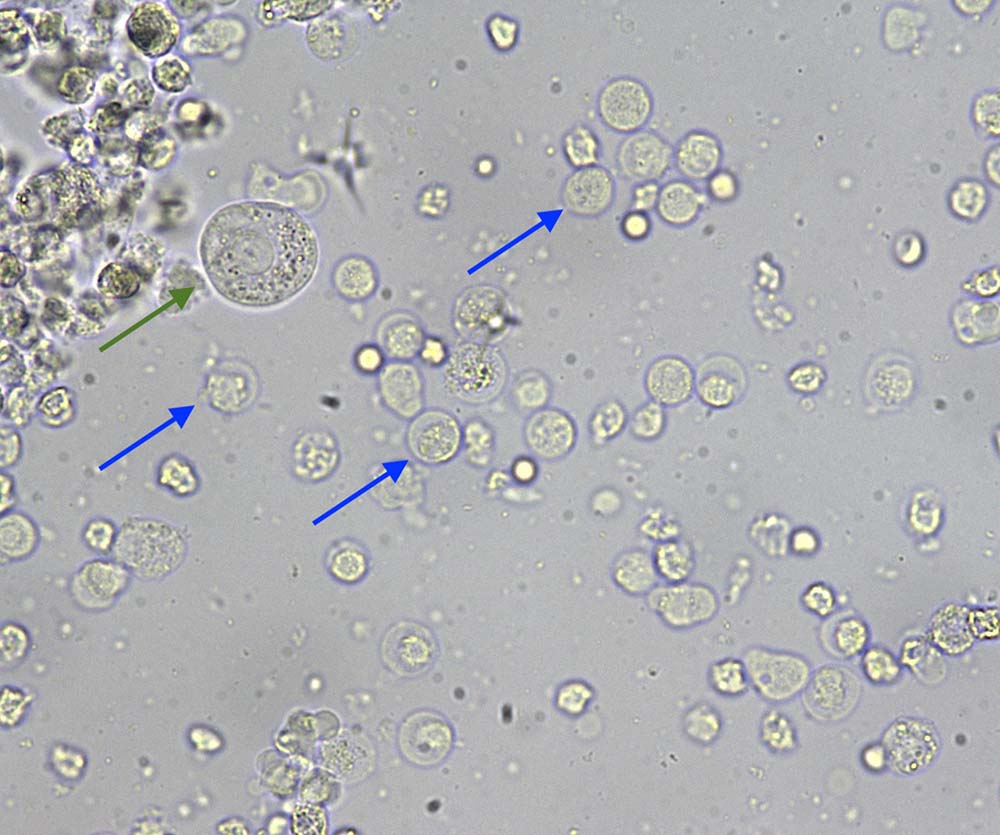
Microscopic examination at high‑power field (40×) of the unstained urine sediment showed a subjective increase (more than five per high-power field) in leukocytes (blue arrows). These appear as large, round structures with a granular appearance, opposite to red blood cells, which are smaller and have a smoother appearance/texture.
On stained sediment, leukocytes are easier to be identified and appear as neutrophils with a typical segmented/lobulated dark purple nucleus. A few epithelial cells (green arrows) and occasional red blood cells are also present. In the stained sample, it is also possible to appreciate the presence of both extracellular and intracellular bacteria – cocci (red arrows).
Cytological diagnosis
A diagnosis of septic neutrophilic inflammation and mild haematuria was determined.
Insight
Urinary tract infection (UTI) is a common diagnosis in companion animal practice. The incidence of UTI in the dog over its lifetime has been reported to be 14 per cent. A large percentage of these infections is caused by bacteria, mostly ascending the external genitalia and urethra, occasionally travelling through the bloodstream, and colonising the urinary tract. Most of the UTIs (approximately 75 per cent) involve a single agent, with Escherichia coli being responsible for up to half of the infections in dogs.
Cytology is a valid instrument for the diagnosis of septic inflammations of the urinary tract in dogs and relies on microscopic findings of sediment examination.
Routine unstained urine sediment examination has been shown to have suboptimal accuracy for detection of bacteriuria, in part due to the presence of small particles (such as pseudobacteria) that resembled bacteria in size, shape and Brownian movement. These particles may have been small lipid molecules, cytoplasmic organelles or amorphous crystals, or debris that did not stain or grow in culture, but contributed to false positive results by use of the routine unstained method of urine sediment examination.
False negative results may also occur, as seen in this case (bacteria not visible in the unstained sediment, but seen in the stained preparation and further confirmed by culture results). Interestingly, the diagnostic accuracy of cytology for the identification on bacteria can be increased by staining the urine sediment smears with Wright-Giemsa‑based stains, which significantly reduce the numbers of false positive and false negative results as shown in a study of more than 400 dogs conducted by Swenson et al (2004).
The International Society for Companion Animal Infectious Diseases’ 2019 guidelines revised the classifications of UTIs and identified the following categories:
Other conditions that should be included in the differential list of infections of the urogenital tract include upper urinary tract infections (pyelonephritis) and bacterial prostatitis in male dogs.
Take-home messages
Bacteria are easier to be correctly identified in Wright-stained urine sediment samples compared with unstained sediment preparations.
However, quantitative bacteriologic culture and antimicrobial susceptibility testing remain the most accurate methods for identifying infection, and selecting effective antimicrobial treatments.
